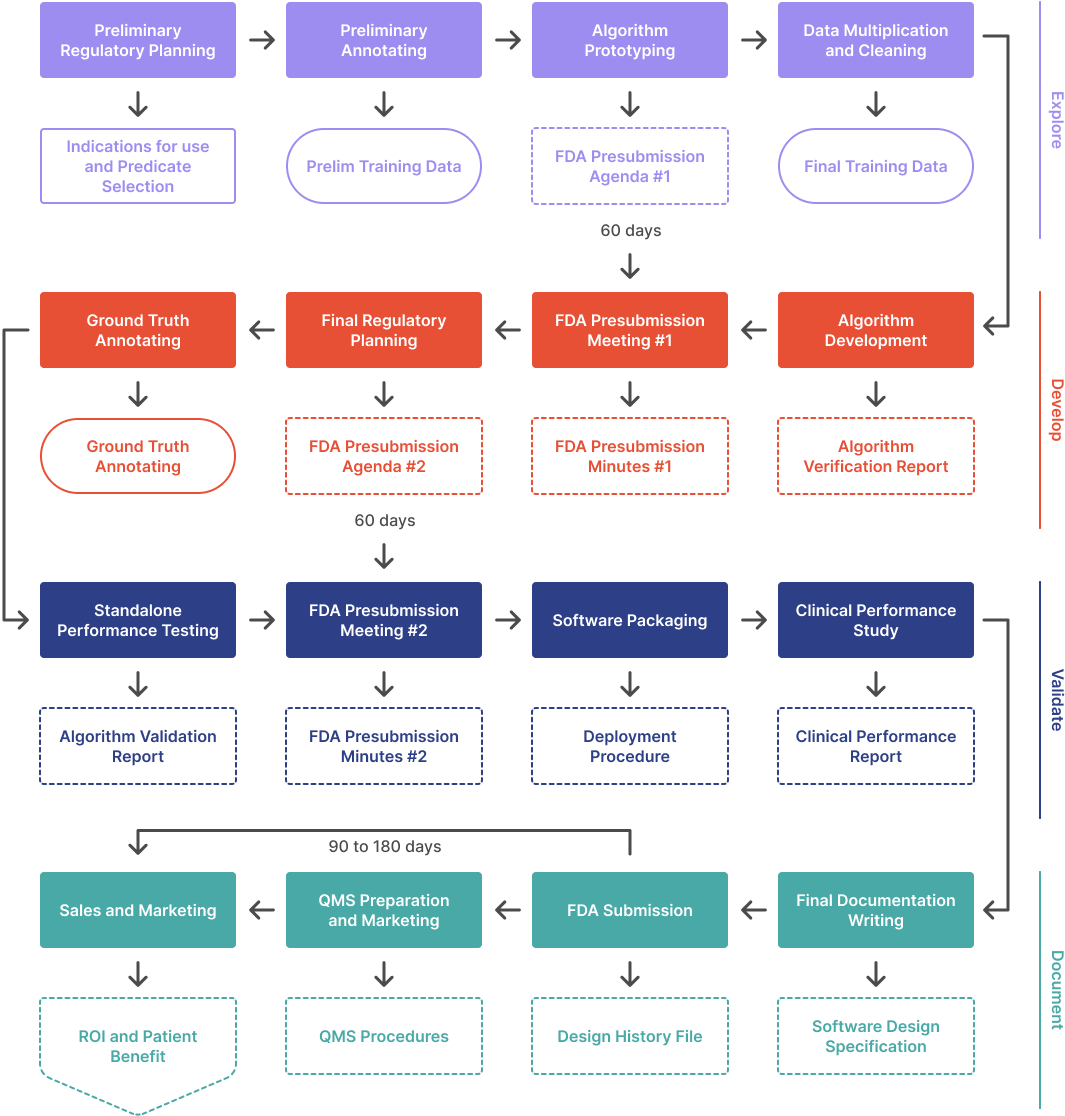
This webinar covers the four phases of the regulatory process: Explore, Develop, Validate, and Document. It also discusses the costs, time, and data requirements involved in the process. Additionally, it provides advice on regulatory strategy, data annotation, and algorithm prototyping.
You may be interested in the companion article, “AI/ML from Idea to FDA”.