Diabeloop’s FDA Clearance: A Landmark Victory for the SaMD Least Burdensome Pathway 🔗
December 22, 2025 – In a move that supports an ongoing shift in the regulatory landscape for digital health, French medical technology company Diabeloop has just secured FDA 510(k) clearance for its DBLG2 algorithm. While the clearance for an automated insulin delivery (AID) system is newsworthy in itself, the real story lies in the strategic regulatory pivot it represents: a successful transition from a hardware-dependent system (SiMD) to a standalone, interoperable software platform (SaMD). This clearance is more than a win for Diabeloop; it’s a powerful proof-of-concept for the entire AI/ML medical device industry, demonstrating that a software-only, least burdensome approach is viable even for high-risk, closed-loop therapeutic applications.
For executives and innovators in the MedTech space, Diabeloop’s journey offers a compelling blueprint for accelerating time-to-market, reducing regulatory overhead, and focusing resources on what truly matters: the intelligence of the algorithm.
The Evolution from SiMD to SaMD: Decoupling from Hardware 🔗
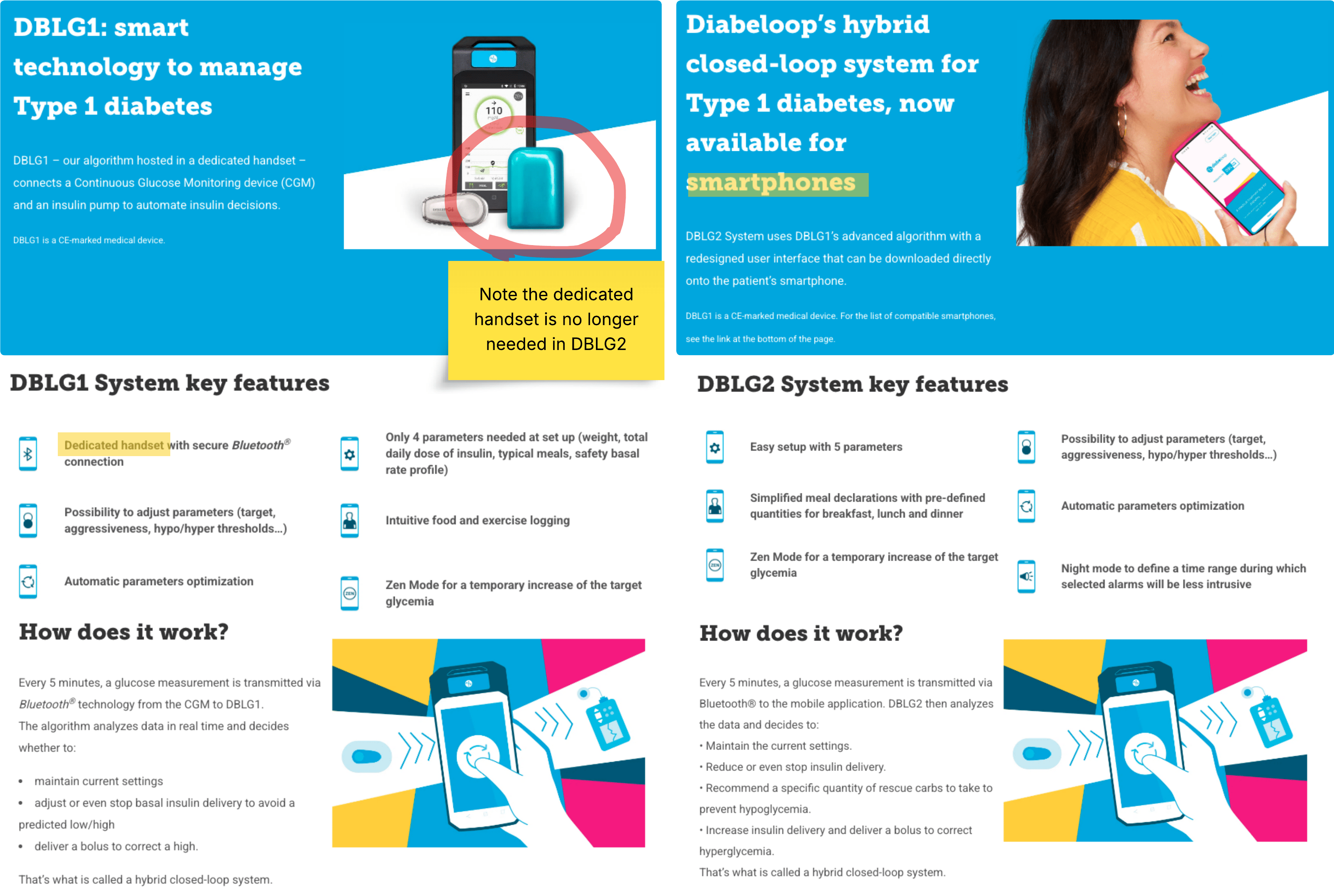
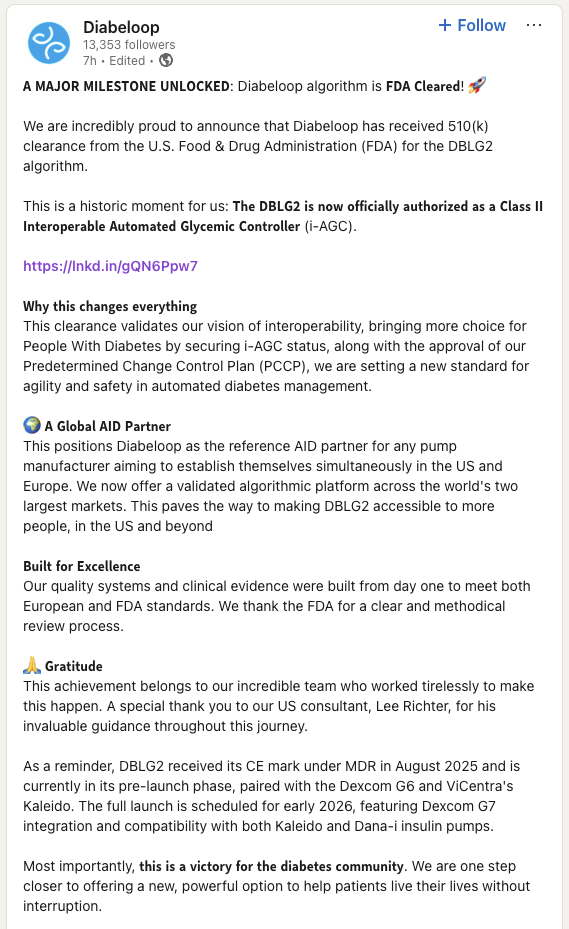
Diabeloop’s first-generation product, the DBLG1 system, was a classic example of Software in a Medical Device (SiMD). The system’s powerful algorithm was hosted on a dedicated, locked-down handset that served as the central hub, communicating via Bluetooth with a continuous glucose monitor (CGM) and an insulin pump. While effective, this model meant the entire system, including the proprietary hardware, was part of the regulated medical device. This subjected the product to the full spectrum of hardware validation requirements, from electrical safety and biocompatibility to supply chain management and manufacturing controls.
In contrast, the newly cleared DBLG2 system is a masterclass in regulatory efficiency. It has been refactored as Software as a Medical Device (SaMD). The core of the product—the AI-driven algorithm—is now encapsulated in a software application that can be downloaded directly onto a patient’s personal smartphone. This seemingly simple change has profound regulatory implications.
As Diabeloop noted in its CE mark announcement for DBLG2, "The application, downloadable directly on the patient’s personal phone after a certifying training, eliminates the need for a dedicated terminal."
By shedding the dedicated handset, Diabeloop effectively decoupled its core intellectual property from the burdens of hardware manufacturing and validation. The smartphone is treated as a general-purpose computing platform, not part of the medical device itself. This strategic pivot allowed Diabeloop to focus its regulatory efforts exclusively on the software, leveraging the FDA’s progressive framework for SaMD.
A New Precedent for High-Risk, Closed-Loop SaMD 🔗
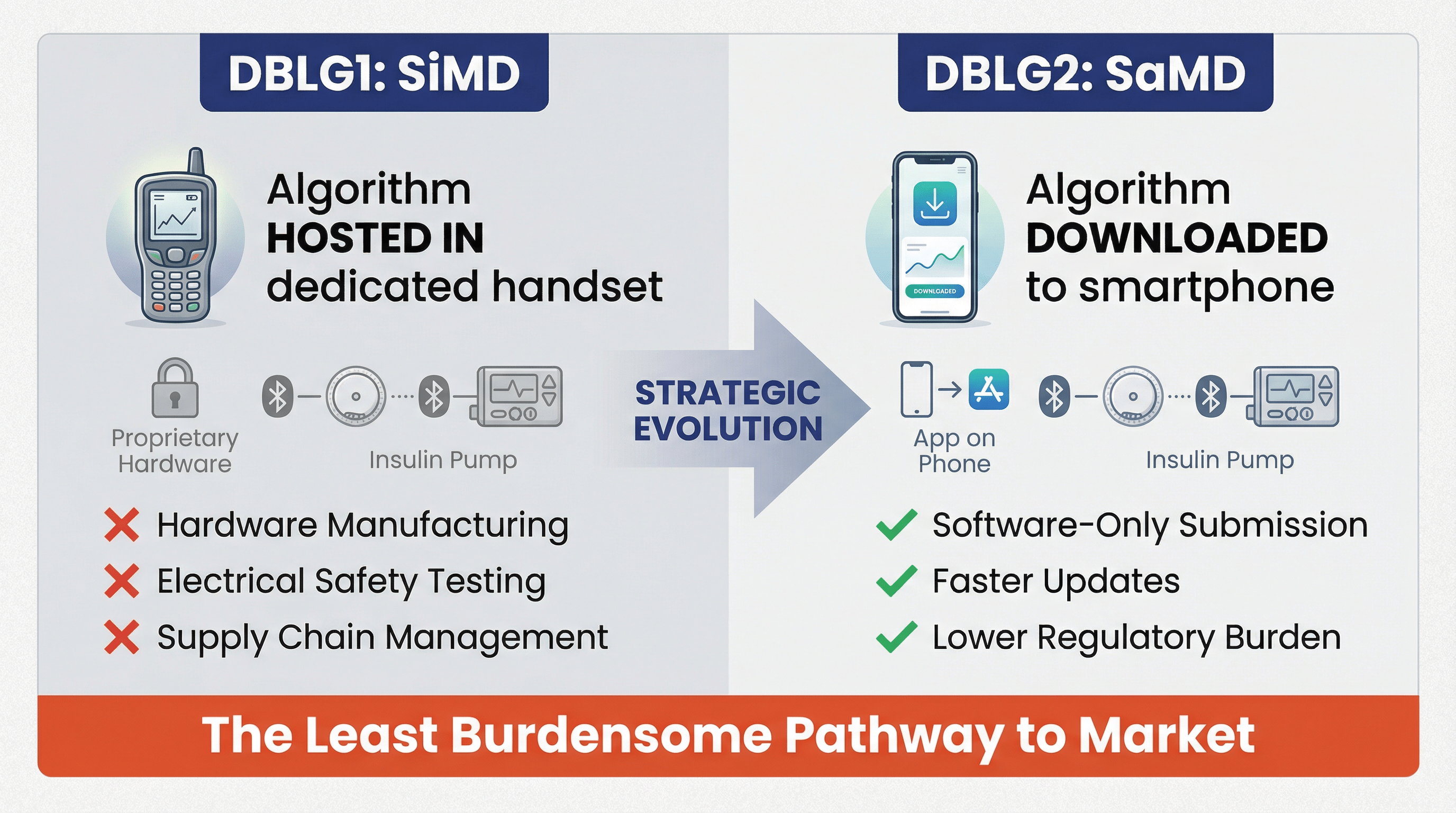
A common misconception is that the SaMD pathway is primarily for lower-risk applications, such as diagnostic aids or data analysis tools. Diabeloop’s clearance shatters this perception. The DBLG2 algorithm operates in a therapeutic closed-loop system where the stakes are incredibly high. An error in the algorithm could lead to incorrect insulin dosing, resulting in severe hypoglycemia or hyperglycemia—both life-threatening conditions.
The FDA’s decision to clear DBLG2 as a Class II interoperable automated glycemic controller (i-AGC) confirms that the agency is confident in the safety and efficacy of SaMD in a control loop for a critical life-sustaining therapy. This sets a crucial precedent for other AI/ML developers working on high-risk applications. It signals that, with robust validation, appropriate special controls, and a clear risk management framework, software can be trusted to make autonomous therapeutic decisions.
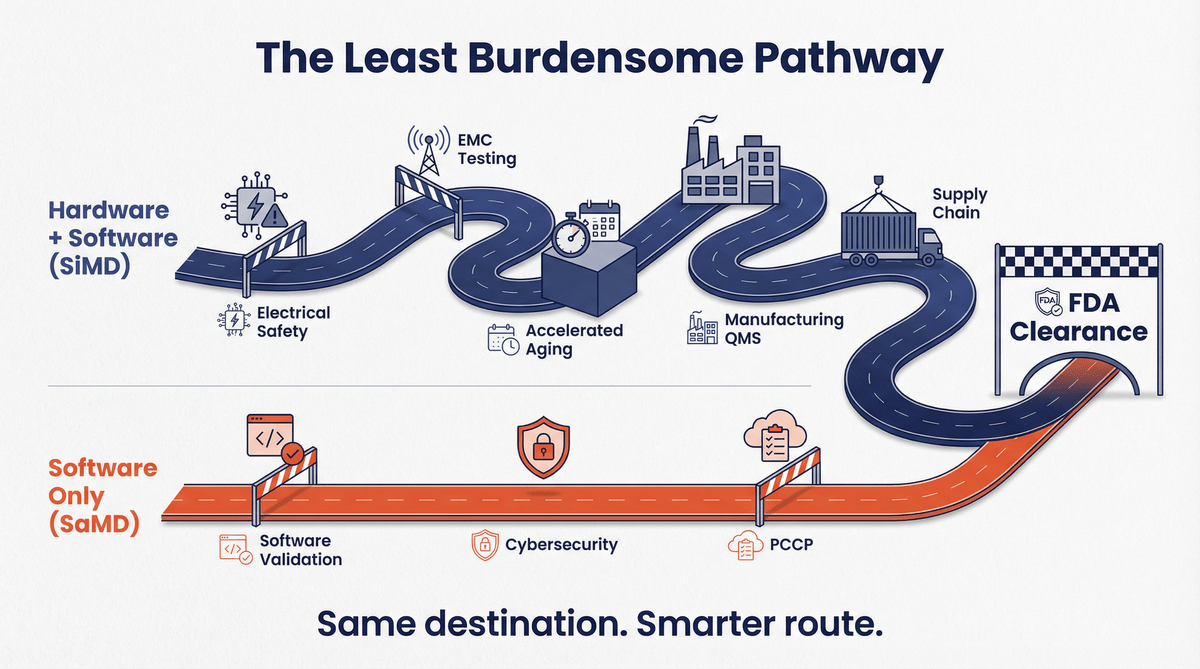
The Least Burdensome Pathway in Action 🔗
For MedTech CEOs and executive teams, the term "least burdensome" is often discussed but rarely seen in such a clear-cut example. The transition from DBLG1 to DBLG2 is a textbook case of finding a more efficient regulatory path.
Hardware is, by its nature, hard. The process of clearing a hardware-based medical device is fraught with complexities that add significant time and cost to a project:
- Electrical Safety & EMC Testing: Rigorous testing to ensure the device doesn’t interfere with other electronics or pose an electrical risk to the patient.
- Biocompatibility: For any patient-contacting materials.
- Accelerated Aging & Durability: Proving the device can withstand years of use.
- Sterilization and Packaging: If applicable, adding another layer of validation.
- Supply Chain & Manufacturing Controls: Establishing and maintaining a QMS for hardware production.
By moving to a SaMD model, Diabeloop sidestepped the majority of these hardware-specific hurdles. Their focus shifted to the core of their innovation: the software itself. This allows for faster development cycles, easier updates (facilitated by the FDA’s Predetermined Change Control Plan, or PCCP), and a dramatically lower barrier to entry for new markets. Additionally, the user experience is better because it is one less thing to carry around and keep charged.
Key Comparison: SiMD vs. SaMD Architecture 🔗
The following table breaks down the fundamental differences between the two systems, illustrating how DBLG2 sheds the hardware dependency of its predecessor.
| Feature | DBLG1 System (SiMD) | DBLG2 System (SaMD) |
|---|---|---|
| Core Architecture | The algorithm is hosted in a dedicated handset. This handset is a piece of proprietary medical hardware. | The algorithm is a standalone mobile application that can be downloaded directly onto a patient's smartphone. |
| Controller Hardware | Requires a dedicated, regulated handset. This is the central piece of evidence for its SiMD classification. | Uses the patient's personal smartphone. The phone itself is not the medical device; it is a general-purpose platform running the SaMD app. |
| Software Delivery | Software is pre-installed and updated on the proprietary handset, managed as part of the medical device. | Software is delivered as a downloadable app, allowing for easier updates and distribution via app stores (pending regulatory approvals). |
| Regulatory Classification | SiMD (Software in a Medical Device). The entire system, including the handset, is the regulated device. This brings hardware validation burdens (electrical safety, manufacturing, etc.). | SaMD (Software as a Medical Device). Only the software application is the regulated medical device. This follows the "least burdensome pathway." |
| "All-in-One" Controller | The dedicated handset is the all-in-one controller. | The smartphone becomes the all-in-one controller, managing the pump, CGM, and loop mode. |
| Data Flow | Glucose data is sent from the CGM to the dedicated handset (DBLG1) for analysis. | Glucose data is sent from the CGM to the mobile application (DBLG2) for analysis. |
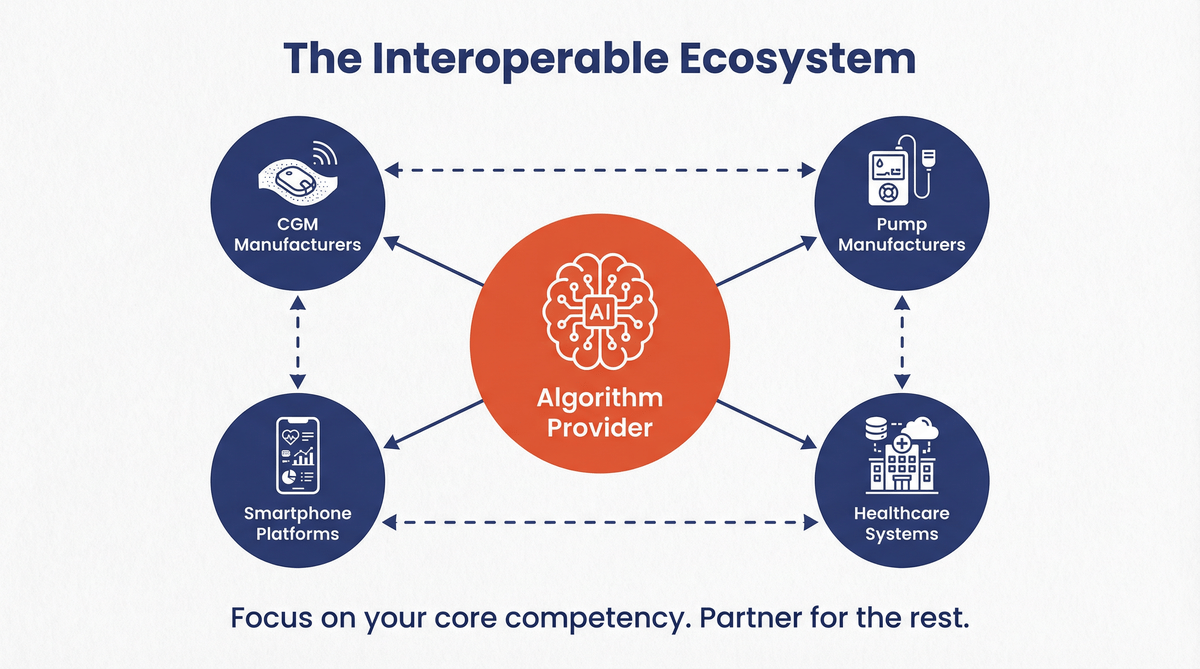
The Future is an Interoperable Ecosystem 🔗
Finally, the i-AGC classification underscores the FDA’s commitment to fostering an interoperable ecosystem. Diabeloop is not manufacturing pumps or CGMs; it is providing the intelligence that connects them. The clearance validates their position as a reference AID partner for any pump or CGM manufacturer that adheres to the interoperability standards.
This is a powerful message for the industry: you don’t have to build everything yourself. Companies can focus on their core competencies and collaborate to bring best-in-class solutions to patients. An AI/ML startup can now realistically aim to develop a cleared therapeutic algorithm that partners with established hardware players, without ever needing to build a factory.
In conclusion, Diabeloop’s DBLG2 clearance is far more than just another algorithm entering the market. It is a strategic masterstroke that provides a clear, actionable roadmap for any AI/ML medical device company. It proves that the SaMD pathway is not only viable but is the most intelligent and least burdensome approach for bringing high-impact, software-driven innovations to patients.
Frequently Asked Questions 🔗
Ready to follow this roadmap? 🔗
Regulatory hurdles shouldn't stall your innovation. Let’s get you FDA cleared. More and more of your competitors are taking the fast lane to market and we don’t want you to be left behind.
Already on the market? You need to protect your market share by accelerating your regulatory clearance process and culture. Hire us for one of our done-for-you services so we can teach your team how to accelerate even faster.
Innolitics guarantees 510(k) clearance if:
- Your algorithm passes our suggested acceptance criteria as we determine during our regulatory strategy and presub service
- And getting FDA clearance is one of the top priorities of your organization
Innolitics guarantees 510(k) submission timeline if :
- Your algorithm is complete and can be containerized or be packaged as an SDK
- And you allow us to project manage the submission and allow us to communicate directly with your engineers
- And getting FDA clearance is one of the top priorities of your organization

