We can partner with you to design, develop, and document software for your medical device. Our team is fully US-Based. Whether you’re looking for support on a special project or a full-service software partner who can get you through the FDA, we can help.
We work on both Software as a Medical Device (SaMD) and Software in a Medical Device (SiMD) products.
We are proud to partner with a diverse array of clients, from visionary startups to industry-leading Fortune 500 companies. Typical clients include:
Clinician Founders
Venture-Backed Startups
Medical Device Companies
Academic Research Institutions
We engage with our clients in three different ways:
Our expertise spans across a broad range of medical-device software applications. We specialize in creating solutions that are not only innovative but also compliant and secure, ensuring they meet the highest industry standards.
We have experience developing a variety of medical device applications, including:
Digital Diagnostics
Medical Image Viewers
Software for IVDs and LDTs
Mobile Medical Apps
Digital Therapeutics (DTx)
Software Accessories
Explore a few of our many case studies:
Prototype → 510(k) → QMS → FDA audit passed. Multi-year partnership with ongoing development.
Dr. Andrew Smith, MD PhD - Radiology

Multi-Year Partnership: CBCT Development → FDA Clearance → Maintenance → Support → M&A Support
Nathan Childress, PhD, DABR, Christof Baltes, PhD - Radiation Oncology
UI design, Custom C++, Qt, Python development, IEC 62304 and FDA software documentation for a successful 510(k) submission
Ryan Shelton, PhD - Ear Nose and Throat

MATLAB to Python port with performance improvements, documentation, and verification
Nick Schmansky - Neurology


Co-Founder at AI Metrics & Chair of Diagnostic Imaging at St. Jude Children’s Research Hospital


Associated VP at Varian Medical Systems

Co-Founder and Chairman of Retrieve Medical


CEO and Co-Founder of PhotoniCare


President and CEO of Mary Bird Perkins Cancer Center

Our engineering work is guided by a clinical perspective. One of our founders is an MD and we have a broad network of clinicians we leverage. We have experience in the following clinical areas:
Radiology
Digital Health
Radiation Oncology
InVitro Diagnostics (IVDs)
Dental
Cardiology
Pre-Clinical
Ear Nose and Throat
Neurology
Gastroenterology and Urology
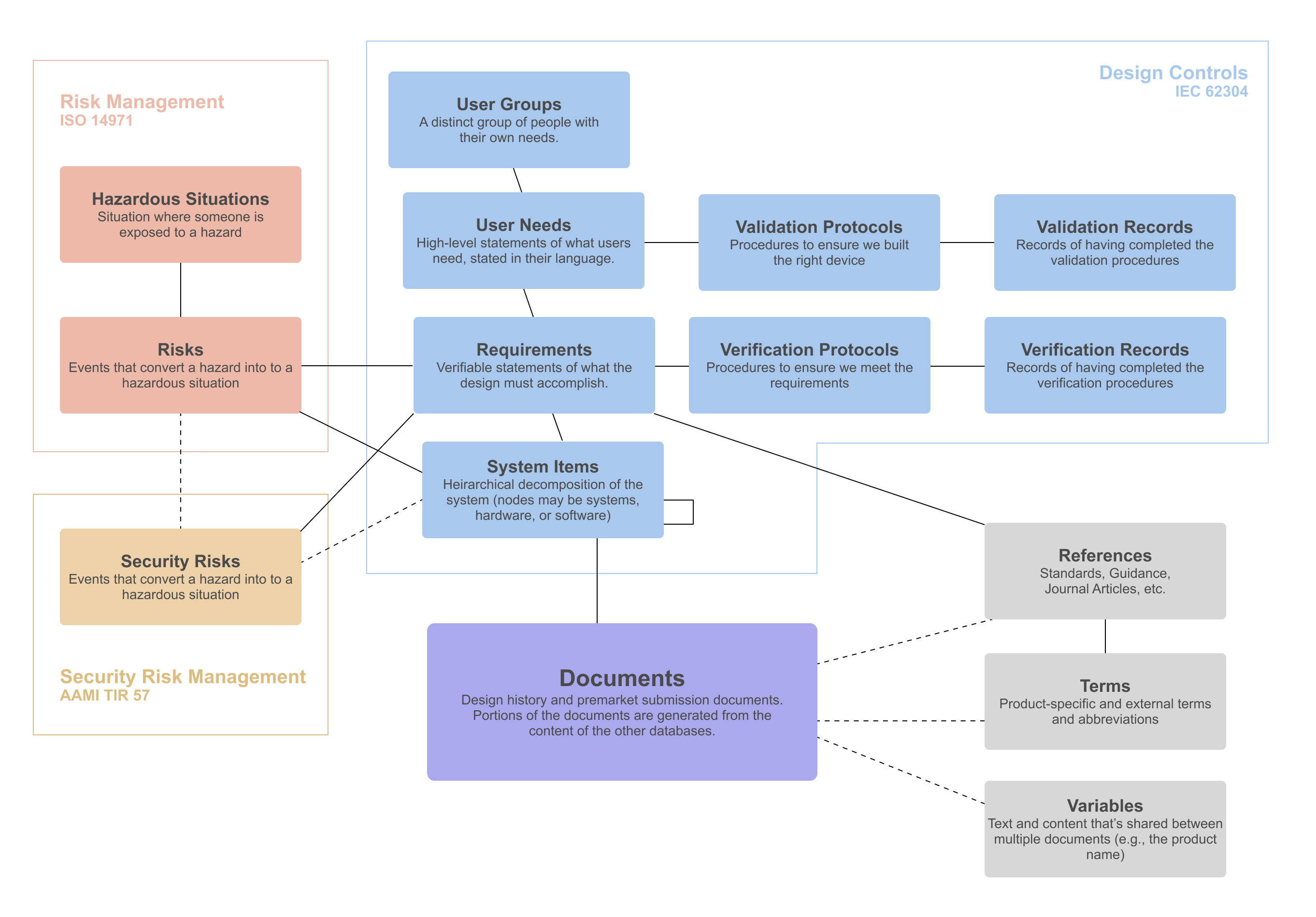
Our ISO 13485 compliant quality management system and development process adheres to the highest standards of quality and compliance, including IEC 62304 for medical device software, ISO 14971 for risk management, and AAMI TIR 57 for cybersecurity. Whether working within our quality management system or integrating with yours, we guarantee excellence and compliance at every stage.
Here are a few articles show-casing our medical-device software expertise:
Every great partnership starts with a conversation. Fill out the form below for a discovery call, and an Innolitics team member will contact you soon.