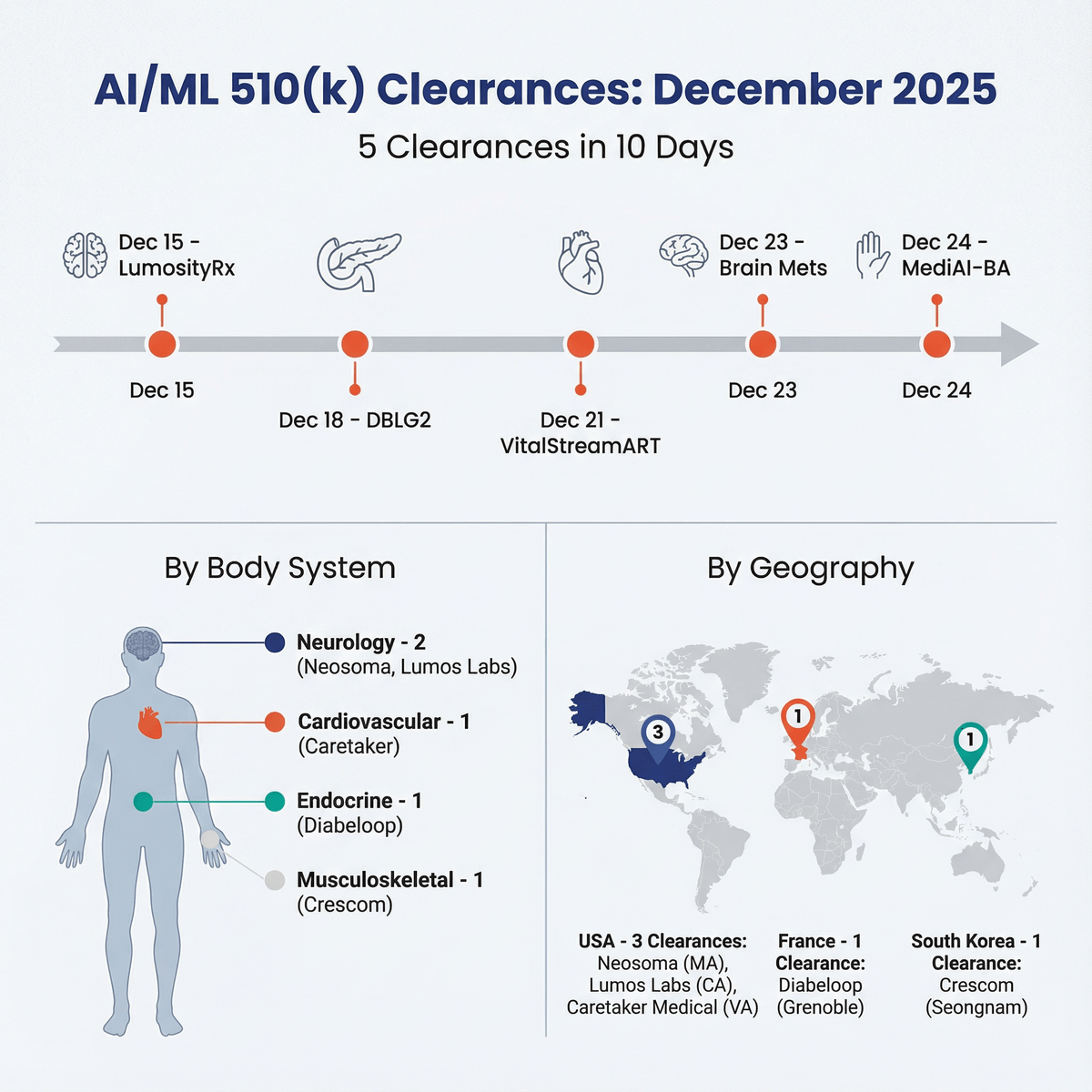
The final weeks of 2025 were marked by a notable series of FDA 510(k) clearances for medical devices incorporating artificial intelligence and machine learning. In a ten-day period, five software-based devices received clearance, indicating continued momentum for AI/ML adoption across several sectors of the healthcare industry.
The clearances encompass a range of applications, including neurology, endocrinology, cardiovascular monitoring, and musculoskeletal diagnostics. The companies behind these devices are based in the United States, France, and South Korea, reflecting the global nature of medical device innovation. The applications vary from diagnostic software that assists clinicians to therapeutic and monitoring platforms.
Summary of Recent AI/ML Clearances 🔗
The following table outlines the key details of these five recent clearances.
| Manufacturer | Device Name | Press Release Date | Indications for Use | Device Inputs | Device Outputs | Performance / Acceptance Criteria | Press Release Link |
|---|---|---|---|---|---|---|---|
| Neosoma, Inc. | Brain Mets | Dec 23, 2025 | AI-based analysis of brain metastases on MRIs | MRI images | Automated lesion identification, measurement, and longitudinal tracking | Not specified | Link |
| Crescom Co. Ltd. | MediAI-BA | Dec 24, 2025 | AI-powered pediatric bone age analysis | Hand and wrist X-ray images | Bone age evaluation and predicted adult height | Specialist-level accuracy (MAD of 0.39 years) | Link |
| Diabeloop | DBLG2 Algorithm | Dec 18, 2025 | Automated insulin delivery for Type 1 Diabetes | CGM data, insulin pump integration | Automated insulin delivery commands | Not specified | Link |
| Lumos Labs | LumosityRx | Dec 15, 2025 | Prescription digital therapeutic for adult ADHD | User interaction with 13 cognitive games | Improvement in sustained and selective attention | 44.2% of users showed clinically meaningful improvement (>1.4 points on TOVA) | Link |
| Caretaker Medical | VitalStreamART | Dec 21, 2025 | Continuous, non-invasive hemodynamic monitoring | Finger sensor or arterial line catheter | Continuous BP, Cardiac Output, SVR, Fluid Response | Not specified | Link |
Analysis of Industry Trends 🔗
These clearances highlight several important trends within the medical device industry. First, the concentration in neurology, with two of the five clearances, suggests a growing focus on applying AI to complex neurological conditions. Neosoma’s Brain Mets software addresses a need in oncology for more efficient tracking of brain metastases, while Lumos Labs’ LumosityRx contributes to the growing category of prescription digital therapeutics (PDTs). The clearance of LumosityRx, supported by a randomized controlled trial, helps to further validate the regulatory pathway for software as a therapeutic intervention.
Second, the clearance of Diabeloop’s DBLG2 algorithm underscores the increasing importance of interoperability. As an interoperable automated glycemic controller (i-AGC), the device is designed to integrate with multiple hardware components, offering greater flexibility to patients and providers. This move toward open systems represents a notable shift in the diabetes technology market. The approval of a Predetermined Change Control Plan (PCCP) for the device also facilitates a more efficient process for future software updates.
Finally, the clearances collectively demonstrate the expansion of AI/ML applications across the continuum of care. The technologies range from diagnostic aids (Neosoma, Crescom) and therapeutic interventions (Lumos Labs, Diabeloop) to continuous monitoring platforms (Caretaker Medical). This indicates a maturation of the field beyond standalone diagnostic tools toward more integrated solutions that can impact the patient journey at multiple stages.
As the industry moves into 2026, these trends suggest continued growth in AI-driven medical technologies.

