Introduction 🔗
Welcome to our article on Digital Therapeutics (DTx), an emerging and transformative field in healthcare. At Innolitics, we are at the forefront of medical device software and regulatory consulting, and we are thrilled about the potential impact of DTx in revolutionizing patient care and treatment outcomes. As this domain rapidly evolves, it's crucial for healthcare professionals, developers, and stakeholders to stay informed about best practices, regulatory landscapes, and the effective implementation of DTx solutions.
Our enthusiasm for DTx stems from its immense potential to enhance patient outcomes, reduce healthcare costs, and democratize access to quality care. At Innolitics, we are excited to help accelerate progress in this field with our combined regulatory and engineering services. From navigating complex regulatory pathways to fine-tuning software development strategies for DTx, our expertise is geared towards empowering innovators and practitioners in this space.
In this article, we will delve into key best practices for developing and implementing DTx solutions, answer frequently asked questions, and provide insights into the regulatory nuances that are crucial for successful market entry and patient impact.
Best Practices 🔗
- Most Digital Therapeutic devices that are regulated by the FDA will require usability studies. Since you will need to complete these studies anyway, and since usability studies help ensure product-market fit, it’s best to run the studies early in the process.
- Engineering teams without experience developing software under the medical devices regulations and standards (IEC 62304, ISO 14971, etc.) should get help from a regulatory/quality group with experience working on Software as a Medical Device (SaMD). SaMD is different than other types of medical devices. It’s most efficient when you engage earlier in the development process—ideally shortly after you prove out the core functionality.
- Most DTx that has been cleared in the US has required a full clinical trial, therefore, when fundraising and planning timelines, DTx startups should plan for these costs.
FAQs 🔗
What is a digital therapeutic device (DTx)? 🔗
A digital therapeutic (DTx) is “health software intended to treat or alleviate a disease, disorder, condition, or injury by generating and delivering a medical intervention that has demonstrable positive therapeutic impact on a patient’s health.”
This definition was adopted by the DTx Alliance in 2023 and is consistent with ISO/TR 11147: Health informatics—Personalized digital health—Digital therapeutics health software systems.
You can read more about the DTx Alliance’s interpretation of this definition here.
As best we’re aware, as of November 2023, FDA has not used the term digital therapeutic or DTx in any of their regulations or guidance.
(Note that the above definition differs slightly from the 2017 definition, which is still found in various places online, including the EU EDPS. Although the two definitions are similar, there are a few clarifications.)
Are all DTx considered medical devices by FDA? 🔗
Many DTx products are considered medical devices in the US, however, some of them may be general wellness devices. For example, there are several general wellness products in the DTx Alliance’s Product Library. You can read more about General Wellness Products in our article, Is it a General Wellness Product or a Medical Device?
Also, keep in mind that some manufacturers may refer to products as being digital therapeutics even if they’re classified as a “general wellness product” by FDA and even if there use doesn’t provide a “demonstrable positive therapeutic impact” on patient health.
Are DTx new? 🔗
Digital Therapeutic (DTx) devices and applications are a relatively new and rapidly evolving area in healthcare, gaining significant traction and recognition over the past decade. The oldest DTx device that I’m aware of is WellDoc’s DiabetesManager System, cleared in 2010 (see the examples).
What is SaMD and SiMD? 🔗
In general, software that is “intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease” is considered a medical device (there are some technicalities that I’m omitting and the definitions vary slightly in different jurisdictions).
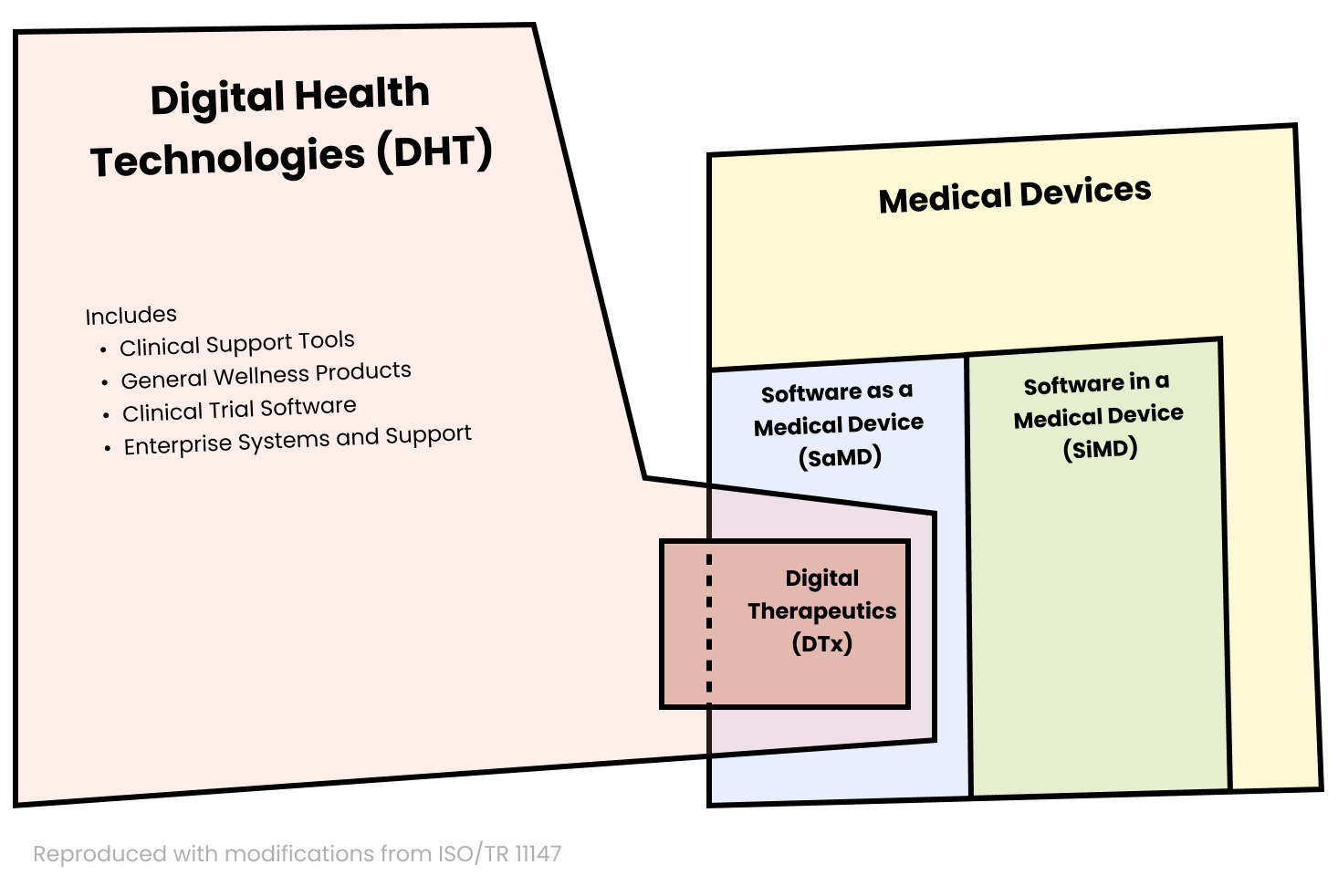
SaMD, or Software as a Medical Device, is software-only medical devices. DTx is a particular type of SaMD.
SiMD, or Software in a Medical Device, is software that’s part of a hardware-based medical device (e.g., an insulin pump).
What is digital health technology (DHT)? 🔗
Digital Health Technology (DHT) is a “system that uses computing platforms, connectivity, software, and sensors for healthcare and related uses.”
DHT is a broad category, which includes a variety of software systems:
- Clinical Trial Management Systems
- Clinical Operations
- Telehealth platforms (e.g., Zoom Healthchare)
- EHRs
- Lifestyle and general wellness apps
- Wearables (e.g., Apple Watch)
- Remote patient monitoring tools
- Digital therapeutics
- Other SaMD and SiMD
What is a PDT? 🔗
PDT stands for prescription digital therapeutic, and it is what it sounds like: a DTx that must be prescribed.
What is the relationship between DTx, DHT, SaMD, and SiMD!? 🔗
The following diagram shows a useful breakdown:
What sorts of clinical evidence is required for DTx? 🔗
Significant clinical evidence is required for most DTx regulatory submissions. For example, several cleared devices have had full clinical trials where one arm of users follow a separate “dummy app” that omits key aspects of the digital therapy.
The 510(k) summaries for devices in your product code will include useful information about clinical evidence that’s been used for similar devices, including in some cases, clinical trial numbers.
Does FDA have any particular regulations for DTx? 🔗
Yes, see the regulations for the product code of your device to see the relevant special controls.
Does FDA have any guidance for DTx? 🔗
No, not as of November 2023.
Note that there is a draft guidance document Digital Health Technologies for Remote Data Acquisition in Clinical Investigations.
Where can I learn more about DTx? 🔗
The Digital Therapeutics Alliance has a lot of useful resources.
Examples 🔗
Product Codes 🔗
In this section, several product codes for DTx devices are presented, along with their special controls. DTx is relatively new and thus we expect there to be a number of De Novo requests that will create new product codes.
PWE Computerized Behavioral Therapy Device For Substance Use Disorders 🔗
| Parameter | Value |
|---|---|
| Name | Computerized Behavioral Therapy Device For Substance Use Disorders |
| Product Code | PWE |
| Description | Computerized behavioral therapy device for psychiatric disorders. |
| Technical Method | The device is a software-based mobile app that provides a computerized version of behavioral therapy to the patient. |
| Regulation | 21 CFR 882.5801 |
| Classification | Class II |
| Identification | A computerized behavioral therapy device for psychiatric disorders is a prescription only device intended to provide a computerized version of condition-specific behavioral therapy as an adjunct to clinician supervised outpatient treatment to patients with psychiatric conditions. The digital therapy is intended to provide patients access to therapy tools used during treatment sessions to improve recognized treatment outcomes. |
| Clinical Validation | Clinical data must be provided to fulfill the following: (i) Describe a validated model of behavioral therapy for the psychiatric disorder; and (ii) Validate the model of behavioral therapy as implemented by the device. |
| Usability | Not explicitly required by the special controls |
| Labeling | (i) Patient and physician labeling must include instructions for use, including images that demonstrate how to interact with the device. (ii) Patient and physician labeling must list compatible devices. (iii) Patient and physician labeling must include a warning that the device is not intended for use as a standalone therapy. (iv) Patient and physician labeling must include a warning that the device does not represent a substitution for the patient's medication. (v) Physician labeling must include a summary of the clinical testing with the device. |
QVO Computerized Behavioral Therapy Device For Insomnia 🔗
| Parameter | Value |
|---|---|
| Name | Computerized Behavioral Therapy Device For Insomnia |
| Product Code | QVO |
| Description | Computerized behavioral therapy device for psychiatric disorders. |
| Technical Method | The device is a software-based mobile app that provides a computerized version of behavioral therapy to the patient. |
| Regulation | 21 CFR 882.5801 |
| Classification | Class II |
| Identification | The device is intended to provide cognitive behavioral therapy to treat insomnia. |
| Clinical Validation | Clinical performance testing must demonstrate that the device performs as intended under anticipated conditions of use, including an evaluation of sensitivity, specificity, positive predictive value, and negative predictive value using a reference method of diagnosis and assessment of patient behavioral symptomology. |
| Usability | Usability assessment must demonstrate that the intended user(s) can safely and correctly use the device. |
| Labeling | (i) Patient and physician labeling must include instructions for use, including images that demonstrate how to interact with the device. (ii) Patient and physician labeling must list compatible devices. (iii) Patient and physician labeling must include a warning that the device is not intended for use as a standalone therapy. (iv) Patient and physician labeling must include a warning that the device does not represent a substitution for the patient's medication. (v) Physician labeling must include a summary of the clinical testing with the device. |
QMY Computerized Behavioral Therapy Device For Treating Symptoms of Gastrointestinal Conditions 🔗
| Parameter | Value |
|---|---|
| Name | Computerized Behavioral Therapy Device For Insomnia |
| Product Code | QMY |
| Description | Computerized behavioral therapy device for treating symptoms of gastrointestinal conditions. |
| Technical Method | The device provides digital therapy either on a website or an app. |
| Regulation | 21 CFR 876.5960 |
| Classification | Class II |
| Identification | A computerized behavioral therapy device for treating symptoms of gastrointestinal conditions is a prescription device intended to provide a computerized version of condition-specific therapy as an adjunct to standard of care treatments to patients with gastrointestinal conditions. |
| Clinical Validation | Clinical data must be provided to fulfill the following: (i) Describe a model of therapy for the indicated gastrointestinal conditions; (ii) Validate the model of therapy as implemented by the device using a clinically defined endpoint; and (iii) Evaluate all adverse events. |
| Usability | Usability assessment must demonstrate that the intended user(s) can safely and correctly use the device. |
| Labeling | (i) Labeling must include instructions for use, including images that demonstrate how to interact with the device; (ii) Patient and physician labeling must list the minimum operating system requirements that support the software of the device; (iii) Patient and physician labeling must include a warning that the device is not intended for use in lieu of a standard therapeutic intervention or to represent a substitution for the patient's medication; (iv) Patient and physician labeling must include a warning to seek medical care if a patient has feelings or thoughts of harming themselves or others; and (v) Physician and patient labeling must include a summary of the clinical testing with the device. |
QWI Computerized Behavioral Therapy Device For The Treatment Of Fibromyalgia Symptoms 🔗
| Parameter | Value |
|---|---|
| Name | Computerized Behavioral Therapy Device For The Treatment Of Fibromyalgia Symptoms |
| Product Code | QWI |
| Description | N/A |
| Technical Method | Computerized Behavioral Therapy |
| Regulation | 21 CFR 876.5960 |
| Classification | Class II |
| Identification | Provides acceptance and commitment therapy (ACT), a form of cognitive behavioral therapy (CBT), for patients with fibromyalgia symptoms. |
| Clinical Validation | Clinical performance testing must demonstrate that the device performs as intended under anticipated conditions of use, including an evaluation of sensitivity, specificity, positive predictive value, and negative predictive value using a reference method of diagnosis and assessment of patient behavioral symptomology. |
| Usability | Usability assessment must demonstrate that the intended user(s) can safely and correctly use the device. |
| Labeling | (i) Labeling must include instructions for use, including images that demonstrate how to interact with the device; (ii) Patient and physician labeling must list the minimum operating system requirements that support the software of the device; (iii) Patient and physician labeling must include a warning that the device is not intended for use in lieu of a standard therapeutic intervention or to represent a substitution for the patient's medication; (iv) Patient and physician labeling must include a warning to seek medical care if a patient has feelings or thoughts of harming themselves or others; and (v) Physician and patient labeling must include a summary of the clinical testing with the device. |
QPF Pediatric Autism Spectrum Disorder Diagnosis Aid 🔗
| Parameter | Value |
|---|---|
| Name | Computerized Behavioral Therapy Device For The Treatment Of Fibromyalgia Symptoms |
| Product Code | QPF |
| Description | Pediatric Autism Spectrum Disorder diagnosis aid. |
| Technical Method | Performs an analysis of patient behavioral data to aid in the diagnosis of Autism Spectrum Disorder. |
| Regulation | 21 CFR 882.1491 |
| Classification | Class II |
| Identification | A pediatric Autism Spectrum Disorder diagnosis aid is a prescription device that is intended for use as an aid in the diagnosis of Autism Spectrum Disorder in pediatric patients. |
| Clinical Validation | Clinical performance testing must demonstrate that the device performs as intended under anticipated conditions of use, including an evaluation of sensitivity, specificity, positive predictive value, and negative predictive value using a reference method of diagnosis and assessment of patient behavioral symptomology. |
| Usability | Usability assessment must demonstrate that the intended user(s) can safely and correctly use the device. |
| Labeling | (i) Instructions for use, including a detailed description of the device, compatibility information, and information to facilitate clinical interpretation of all device outputs; and (ii) A summary of any clinical testing conducted to demonstrate how the device functions as an interpretation of patient behavioral symptomology associated with Autism Spectrum Disorder. The summary must include the following: (A) A description of each device output and clinical interpretation; (B) Any performance measures, including sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV); (C) A description of how the cutoff values used for categorical classification of diagnoses were determined; and (D) Any expected or observed adverse events and complications. (iii) A statement that the device is not intended for use as a stand-alone diagnostic. |
Devices 🔗
WellDoc DiabetesManager System 🔗
First clearance in 2010 (https://www.accessdata.fda.gov/cdrh_docs/pdf10/K100066.pdf). Some may argue that it’s not a true DTx.
Included a human factors study.
Cognoa ASD Diagnosis Aid 🔗
From the FDA Breakthrough Device press release in June, 2021:
The Cognoa ASD Diagnosis Aid is a software as a medical device that uses a machine learning algorithm to receive input from parents or caregivers, video analysts and health care providers to assist physicians evaluate a patient at risk of ASD. The device consists of three main components: a mobile app for caregivers and parents to answer questions about behavior problems and to upload videos of their child; a video analysis portal that allows manufacturer-trained and certified specialists to view and analyze uploaded videos of patients; and a health care provider portal that is intended for a health care provider to enter answers to pre-loaded questions about behavior problems, track the information provided by parents or caregivers and review a report of the results. After processing the information provided by parents, caregivers and healthcare providers, the ASD Diagnosis Aid reports a positive or negative diagnosis if there is sufficient information for its algorithm to make a diagnosis. If there is insufficient information to render a “Positive for ASD” or “Negative for ASD” result to help determine a diagnosis, the ASD Diagnosis Aid will report that no result can be generated.
Although it seems to me that it is not DTx, since it is diagnostic, several academic papers have referred to this device as being DTx so I’ve included it here. Here are some comments about the clinical study that was run for the device:
The FDA assessed the safety and effectiveness of the Cognoa ASD Diagnosis Aid in a study of 425 patients aged 18 months through 5 years in 14 different clinical care sites, with an average age of 2.8 years. The study compared the assessments made by the device directly against the assessments made by a panel of clinical experts who used the current standard ASD diagnostic process. The device provided a “Positive for ASD” or “Negative for ASD” result to aid in making a diagnosis in 32% of patients. For those with a “Positive for ASD” or “Negative for ASD” result, the device results matched the panel’s conclusions for 81% of patients who tested positive for ASD by the device and 98% of patients who tested negative for ASD by the device. In addition, the device made an accurate ASD determination in 98.4% of patients with the condition and in 78.9% of patients without the condition.
Trial for reSet Substance Use Disorder DTx 🔗
https://clinicaltrials.gov/study/NCT01104805
Trial for Regulora Gastrointestinal DTx 🔗
The Regulora “Computerized Behavioral Therapy Device For Treating Symptoms of Gastrointestinal Conditions” device underwent NCT04133519 The Efficacy and Safety of IBS Digital Behavioral Treatment Study. Here are some details:
| Parameter | Value |
|---|---|
| Summary | A Randomized, Double-Blind, Comparator-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of Two Self-administered behavioral treatments for Adult Subjects with Symptomatic Irritable Bowel Syndrome (IBS). |
| Arm 1 | Active behavioral Treatment Arm (Regulora; Gut-Directed Hypnotherapy Software as a Medical Device - SaMD) |
| Arm 2 | Active Comparator behavioral treatment arm (MR-1; Muscle Relaxation, Software as a Medical Device - SaMD) |
| Enrollment | 378 |
| Inclusion Criteria | Provision of signed and dated informed consent form Stated willingness to comply with all study procedures and availability for the duration of the study Male or female, aged 18-70 Confirmation of the IBS and IBS subtype diagnosis by a study site physician using Rome IV diagnostic criteria Possess an iPhone Operating System (iOS) Apple or Android smartphone or iOS tablet (iPad) released in 2015 or later Agreement to input information about their abdominal pain and bowel movements on a daily basis into Curebase software Agreement to have their anonymized data stored in the cloud for up to 2 years after the conclusion of the study, and to have the data used for research purposes. Agreement to maintain stable dosage of IBS medications during the course of treatment and not to add new IBS medication or stop current IBS medications unless directed to do so by the participants treating physician. Changes in treatment will be captured using a concomitant medication assessment. Average "Worst Daily Pain Severity" of >3 on a 11-point numeric rating scale (NRS) over the full 28-day pre-treatment symptom tracking period Consistent submission of Pain Severity scores via the Curebase app (data submitted on 80% or more of days in the symptom tracking window) |
| Exclusion Criteria | Evidence of current structural intestinal abnormalities that better explain the participant's IBS symptoms (e.g., celiac disease, inflammatory bowel disease - Crohn's Disease and ulcerative colitis, prior abdominal surgeries such as weight loss surgery or bowel resection) Medication use, other illnesses or conditions that can explain their gastrointestinal symptoms e.g.,regular narcotic use or dependency, Over The Counter (OTC) stimulant laxative dependence (i.e, progressively larger doses of Senna or Bisacodyl containing compounds are needed to produce a bowel movement), history of radiation to the abdomen. Diagnosed and/or treated for a malignancy within the past 5 years (other than localized basal or squamous cell carcinomas of the skin) Current psychotherapy, hypnotherapy, or cognitive behavioral therapy (CBT) for IBS Inability to commit to completing all treatment sessions Have an unstable extraintestinal condition whose immediate or foreseeable treatment needs would realistically interfere with study demands, e.g., ability to participate in online treatment sessions or follow daily diary. Active psychiatric disorder (e.g., post-traumatic stress disorder, depression associated with high risk of suicidal behavior, psychotic or delusional disorders, dissociative disorders, or gross cognitive impairment) Subjects that report a current gastrointestinal infection or an infection within the 4 weeks prior to the evaluation that would otherwise obscure IBS symptoms. In cases of gastrointestinal infection baseline evaluation will be delayed a minimum of 4 weeks until after complete recovery. Current or recent use of a gut-targeted antibiotic such as Neomycin or Rifaximin during the 12 weeks prior to baseline assessment. In the case of treatment with rifaximin or neomycin, eligibility will be suspended for 12 weeks from the initial date of use. Any condition that an investigator feels may interfere with the conduct of the study |
Revision History 🔗
| Date | Summary of Change |
|---|---|
| 8 December 2023 | Initial Version; It’s still rough around the edges, but useful enough to publish. |

