Best Practices 🔗
- BDD’s are much more difficult to get than they used to be. You usually need to have a significant amount of clinical evidence.
- Consider doing a pre-sub for your break through (see ).
- Try to make the case that you hit all four of the eligibility sub-items for criterion two.
The BDD Program 🔗
What is a Breakthrough Device Designation? 🔗
The Breakthrough Device Designation, or BDD, is a pre-market program for high-impact medical devices.
It provides certain medical device manufacturers with prioritized review of their submissions and increased interaction with FDA during product development. The program is designed to help patients have more timely access to devices that may provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. Manufacturers must apply for and receive the designation before submitting their marketing application.
What are the benefits of a BDD? 🔗
Being accepted to the BDD program has several benefits.
- It helps with the marketing clearance from the FDA.
- It can help entice investors.
- It opens new reimbursements pathways.
How does a BDD help with marketing clearance? 🔗
- Provides “expedited review”, although the reality is more nuanced. For low to moderate risk devices, it appears the BDD program can actually slow you down.
- Provides you frequent interaction with FDA in the form of 45-day-long sprints. This is opposed to 60 - 75 day waits for pre-subs.
- FDA will agree to a clinical protocol design in writing.
How does a BDD help with reimbursement? 🔗
A BDD can help with several CMS reimbursement pathways:
| Pathway | Pathway Description | Impact of a BDD |
|---|---|---|
| Transitional Coverage for Emerging Technologies (TCET) | A new program designed to expedite national coverage of breakthrough devices. Manufacturers work with CMS to generate evidence. | Only devices with BDD are eligible! |
| New Technology APC | A service is paid under a New Technology APC until sufficient claims data have been collected to allow CMS to assign the procedure to a clinical APC group that is appropriate in clinical and resource terms. |
Having a BDD can help with the process to get an APC assignment. |
| New Technology Add-On Payment (NTAP) | A special payment methodology for devices used in inpatient procedures. When hospitals use the device, Medicare will make additional payments in some cases. | Devices with BDD don’t need to demonstrate the Substantial Clinical Improvement criterion. |
What are the consequences if you don’t have the BDD granted? 🔗
Nothing other than wasted time.
FDA will not make the BDD public, so you don’t need to announce it to anyone.
You may re-apply once you have new data.
What devices are eligible for a BDD? 🔗
The device needs to require a 510(k), De Novo, or PMA—i.e., it can’t be a general wellness device or a 510(k) exempt device. You also can’t have submitted a marketing application already.
Then there are the statutory criteria you need to meet:
- First Criterion: Must provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions
-
Second Criterion: Meets one of the following:
- Represents a breakthrough technology
- No approved or cleared alternative exists
- Offers significant advantages over existing approved or cleared alternatives (e.g., eliminated need for hospitalization, improve quality of life, enable self-directed care, establish long-term clinical efficiencies, etc.)
- The availability of the device is in the best interest of patients
In practice though, try to hit all four of the Second Criterion sub-bullets, not just one of them.
When does it make sense to apply for a BDD? 🔗
We typically don’t always suggest it for low to moderate risk devices, even if they meet the criteria, since the extra interaction and scrutiny with FDA often doesn’t justify the benefits. However, it really depends on whether it will help with your reimbursement strategy or fundraising.
It is also helpful if you need a flexible clinical study design or would like FDA to agree (in writing) to a clinical study protocol for an expensive study.
How do you apply for a BDD? 🔗
A BDD is like an in-depth pre-sub. The typical application is 20-50 pages depending on how much data you have.
Here is the overall process:
- Create Designation Request (see Appendix 1 of the guidance for an example)
- Wait 30 days
- FDA grants it or requests additional information
- Wait up to another 30 days
- FDA grants or denies
What content goes in a Designation Request? 🔗
The FDA recommends that your designation request include:
- information to describe the device,
- the proposed indication for use,
- regulatory history,
- how your device meets the statutory criteria for a Breakthrough Device, and
- what type of marketing submission you plan to submit to the FDA for your device.
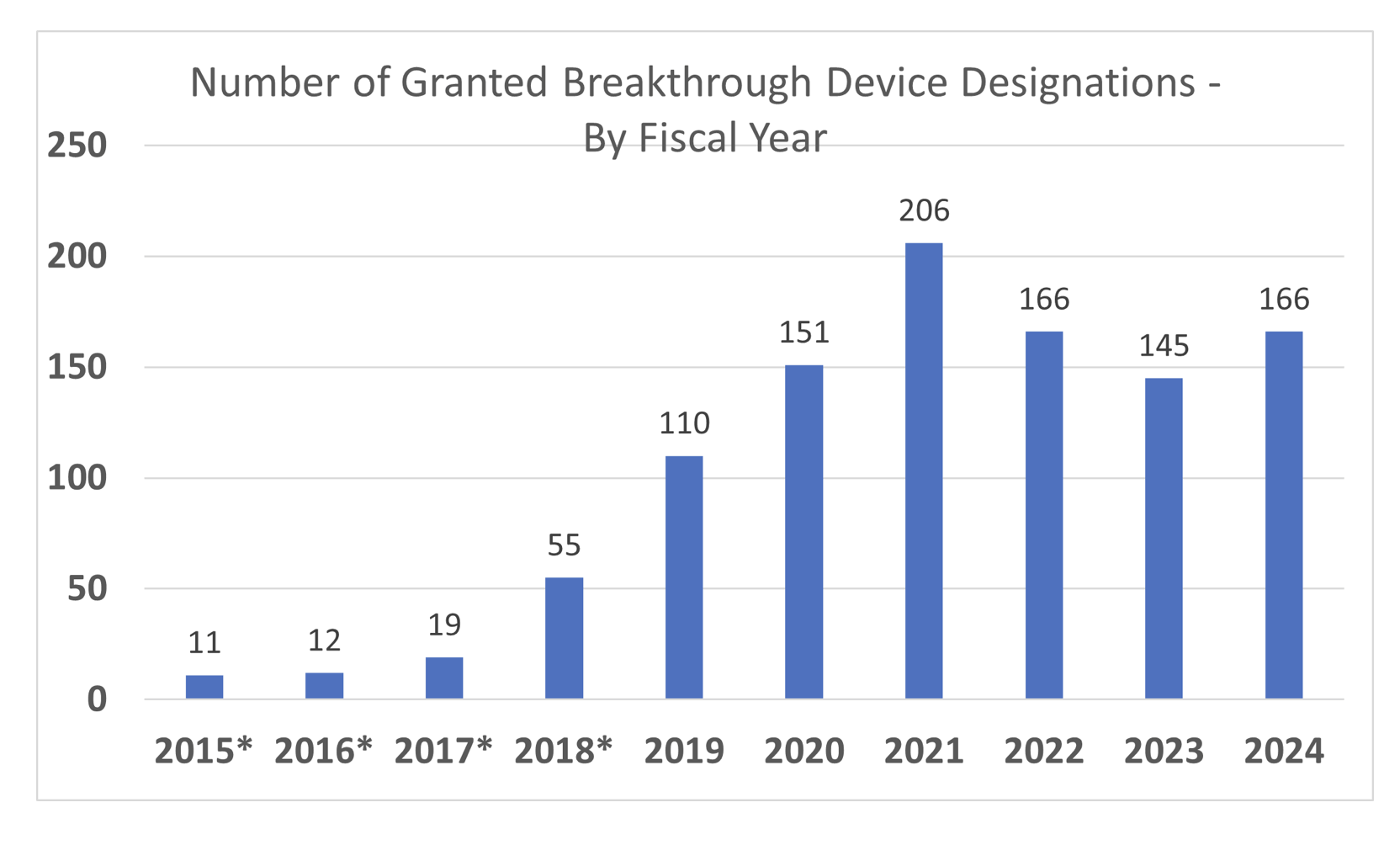
How common are BDDs? 🔗
This graph is taken from the FDA’s FAQ webpage:
Where can I learn more about the BDD program? 🔗
- Statutes
- Regulations
- None
- Guidance
- Other
- FDA’s Breakthrough Device Program FAQ and Webpage which also includes a list of all the breakthrough devices that have marketing authorizations.
- A statistical analysis of non-public BDD data showing the review times of BDDs by therapeutic area and review team.
The STeP Program 🔗
What is the STeP Program? 🔗
The Safer Technologies Program (STeP) is a voluntary program for medical devices that can significantly improve the safety of existing treatments or diagnostics. Unlike the Breakthrough Devices Program, STeP is for devices targeting less serious conditions that are non-life-threatening or reasonably reversible.
STeP aims to expedite development and review of these devices while maintaining FDA's standards for premarket approval, 510(k) clearance, and De Novo marketing authorization.
As of May 2025, my personal opinion is that the STeP program will be deprioritized after the FDA downsizing, as (unlike the BDD program) it is not a statutorily approved program. That said, there’s no explicit evidence this is the case to date.
What devices are eligible for the STeP program? 🔗
The device needs to require a 510(k), De Novo, or PMA—i.e., it can’t be a general wellness device or a 510(k) exempt device. You also can’t have submitted a marketing application already.
Then there are the criteria you need to meet:
- First Eligibility Factor: Not eligible for the Breakthrough Devices Program due to the less serious nature of the disease or condition treated, diagnosed, or prevented by the device
-
Second Criterion: Should be reasonably expected to significantly improve the benefit-risk profile of a treatment or diagnostic through substantial safety innovations that provide for at least one of the following
- A reduction in the occurrence of a known serious adverse event
- A reduction in the occurrence of a known device failure mode
- A reduction in the occurrence of a known use-related hazard or use error
- An improvement in the safety of another device or intervention
How useful is the STeP program? 🔗
Similar to the BDD program, we’ve had clients present mixed results. In some cases, the extra communication with FDA actually can be a determinant and can slow the process down.
Where can I learn more about the STeP program? 🔗
- Guidance
- Other
Revision History 🔗
| Date | Author | Changes |
|---|---|---|
| 2025-02-12 | David | Initial Version |

