Best Practices 🔗
One of the very first document I begin with when I start to create a 510(k) is the Device Description. Besides being very important, it gets the ball rolling. It also sets the tone and rhythm of the entire submission because it is one of the first documents the FDA digs into when they do their deep dive. I believed that Innolitics’ DYI 510(k) Accelerator provides a near perfect example of what a Device Description should be.

In this article, I will give my tips and lessons I have learned over the course of my career. But more importantly I will provide my review and commentary of the Device Description contained within Innolitics’ DYI 510(k) Accelerator. My hope is that you will be encouraged to consider the value that Innolitics is offering. By obtaining this tool, you will avoid mistakes and traps which can delay your 510(k).
Generally, there are 10 elements to any Device Description. They are:
- Trade name
- Description
- Indications for Use
- Contraindications
- Proposed Conditions of Use
- Intended Patient Population
- Intended User
- Principle of Operation
- Images of the Device
- Conclusion
For the sake of brevity, I will combine some of the elements for my explanation of what constitutes a good Device Description for a 510(k), and then I will provide my professional critique of how Innolitics’ proven approach in creating their Device Description can help you accelerate your 510(k) Project.
Basically, the Device Description is a narrative. Technical writing is very different then writing a creative essay. While it can seem boring, there are techniques you can employ which will capture your reader’s attention to achieve your ultimate objective which is a 510(k) clearance. With Innolitics’ DYI 510(k) Accelerator, which features an actual AI/ML SaMD that was recently cleared by FDA, you will be stepping on road to success.

When I begin to write, I always start with an outline as described above. I will also pretend that I am actually in the same room with the FDA Reviewer. I constantly keep in mind my audience. When the FDA opens up your device description, that person is trying to understand your device. At this juncture, the FDA is not skeptical or even critical. You should not begin with trying to impress the FDA. Rather you should keep it simple and clear. I actually pretend to listen to the questions that the FDA would be asking while they are reading my writing. I find that this technique helps me to concentrate and to remain focused.

Based upon my record of successful submissions to the FDA, I believe that the FDA truly appreciates documents that are organized and easy to understand. The life of a FDA Scientific Reviewer is not easy. Their calendar is blocked full of demands and deadlines such as 510(k) submissions, PMA Supplements, IDE applications, and Q-SUB Meetings, etc. With that being said, the Device Description, which is located with Innoltics’ DIY 510(k) Accelerator, does a very good job of illustrating this principle. It is functions as both an example and a template.


I am a great believer in templates. Not only do they allow you to work more efficiently, but they also help you be consistent. Consistency when writing a device description is extremely important. Elements of your description will appear throughout your submission such as in the User Manual, 510(k) Summary, and the Substantial Equivalence Discussion. If you are collaborating with other team members in the preparation of your 510(k) submission, it is imperative that the way in which the device is described in one section is the same throughout your submission. Otherwise, the FDA will pick up upon this, and it can result in a 510(k) hold. Innoltics’ DIY 510(k) Accelerator will help your team to function consistently.
For example, on the right side of the ESTAR Template of the DIY 510(k) Accelerator, you can quickly open the attachments and ascertain that the description of a device is consistent through the submission. This is a great way that the DIY 510(k) Accelerator can be used as a tool when training your team at the beginning of a 510(k) project.
After describing your device within your Device Description, the next section is the indications for use/intended use. This can be confusing to some. The indications for use need to state what your device is being used for. Usually, the indications for use statement is a derivative of your predicate device(s) with slight modifications. I could write pages on the subject, but for this article, the most important concept is they come right after your description of your device.
The template featured in the DIY 510(k) Accelerator does this for you. The only thing that I would be careful of would be- to not mix the intended use with the indications for use. As a rule, the intended use is very general, and the indications for use are more precise and very specific. Some companies use them together for reasons such as for ISO/MDR compliance. Nevertheless, the indications for use need to be exact throughout your 510(k) submission. The intended use, if you should include it cannot exceed, or enhance your indications for use. Instead, they should complement them.
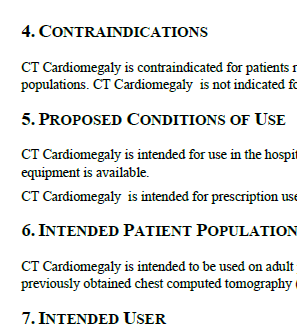
What I find novel about Innoltics’ DIY 510(k) Accelerator is that it has a section for Contraindications, the Proposed Conditions of Use, the Intended Patient Population, and the Intended user. While this information is located in the Instructions for Use or User Manual, I like how their template and their example prompts you to also include that information in the Device Description. This is a good thing because it helps the FDA at this preliminary stage of review, to not have to jump to the labeling section. Once again, anything that helps the FDA in their review by making their job easier, helps you. Just make sure that these statements agree with your labeling and other documents, otherwise you will get a hold letter from the FDA.
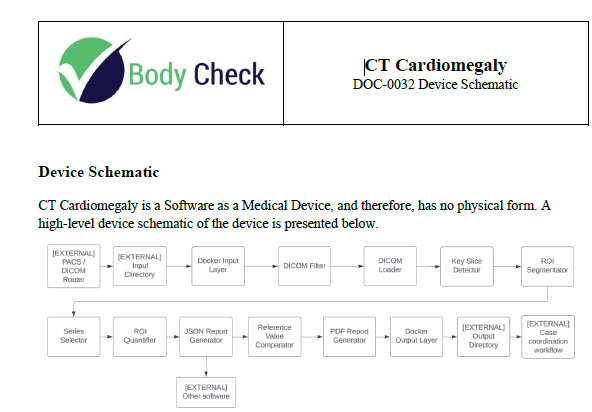
The next section that needs to be included in the Device Description for a 510(k) submission is the Principle of Operation of your device. At this stage, the device should be described from an input/output perspective. For example, how does the device provide its therapeutic effect? Or how does your device provide information so that a Heathcare Professional can make a decision based upon that information?
If your product is physical, then images of the device are useful. In the case of software, then you must become creative and rely upon a combination of block diagrams and schematics. If your software device provides a report, then include a sample of that report. Or if an output is provided in a GUI (graphical user interface) display, then provide a snapshot of the screen. Once again, the template that you will have access to when you purchase Innolitics’ DIY 510(k) Accelerator is not only ready to use, but it is customizable to your medical device.
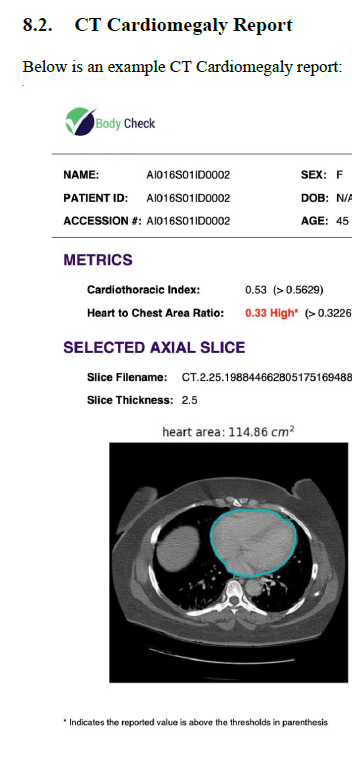
Over the coming weeks, I will be writing more articles about Innoltics’ DIY 510(k) Accelerator along with my advice and tips for creating 510(k) Submission. Meanwhile, I urge you to take advantage and purchase Innoltics’ DIY 510(k) Accelerator because it is an incredible product which will save your company time and money. By doing so, you will avoid the rough road that can delay your 510(k) journey.

Frequently Asked Questions 🔗
Revision History 🔗
| Date | Changes |
|---|---|
| 2024-05-24 | Initial Version |


