Innolitics introduction 🔗
Innolitics provides US FDA regulatory consulting to startups and established medical-device companies. We’re experts with medical-device software, cybersecurity, and AI/ML. See our services and solutions pages for more details.
We have practicing software engineers on our team, so unlike many regulatory firms, we speak both “software” and “regulatory”. We can guide your team through the process of writing software validation and cybersecurity documentation and we can even accelerate the process and write much of the documentation for you (see our Fast 510(k) Solution).
About this Transcript 🔗
This document is a transcript of an official FDA (or IMDRF) guidance document. We transcribe the official PDFs into HTML so that we can share links to particular sections of the guidance when communicating internally and with our clients. We do our best to be accurate and have a thorough review process, but occasionally mistakes slip through. If you notice a typo, please email a screenshot of it to Mihajlo at mgrcic@innolitics.com so we can fix it.
Preamble 🔗
Document issued on January 13, 2025.
Draft document issued on November 3, 2003.
For questions about this document regarding CDRH-regulated devices, contact ORP: Office of Regulatory Programs/DRP1: Division of Submission Support at (301) 796-5640 or by e-mail at OPEQSubmissionSupport@fda.hhs.gov. For questions about this document regarding CBERregulated devices, contact the Office of Communication, Outreach, and Development (OCOD) at 1-800-835-4709 or 240-402-8010, or by email at ocod@fda.hhs.gov.
Contains non-binding guidance.
I. Introduction 🔗
The purpose of this guidance document is to provide industry and FDA staff with information regarding the Premarket Approval Application (PMA) and Humanitarian Device Exemption (HDE) modular review program and to outline the procedures for submitting or reviewing a modular PMA or HDE.
Throughout this guidance document, the terms “we,” “us” “our” refer to FDA staff from the Center for Devices and Radiological Health (CDRH) or Center for Biologics Evaluation and Research (CBER). “You” and “your” refers to the submitter.
In general, FDA’s guidance documents do not establish legally enforceable responsibilities. Instead, guidances describe the Agency’s current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.
II. Background 🔗
In a traditional PMA1 or HDE2, the applicant submits all data, as outlined in 21 CFR 814.20 for PMAs and 21 CFR 814.104 for HDEs, at the same time, regardless of when testing is completed. FDA begins its review only upon receipt of all the required information. In 1998, FDA introduced an alternative regulatory pathway, which allowed a modular approach to PMA submissions. On October 26, 2003, the Medical Device User Fee and Modernization Act of 2002 (MDUFMA), Public Law 107-250 was enacted. Section 209 of MDUFMA amended the Federal Food, Drug and Cosmetic Act (FD&C Act) to codify the modular review approach.3 As stated in the guidance, applicants can also apply this modular approach to HDEs.
The modular review approach is intended to provide a mechanism by which applicants may submit non-clinical data and manufacturing information for review while still collecting, compiling, and analyzing the clinical data using the final device design. Therefore, a modular PMA or HDE is a compilation of sections or "modules" submitted at different times that together become a complete application. The goal of this approach is to increase the efficiency of the review process by allowing applicants to submit discrete modules of the application to FDA for review soon after completing the non-clinical testing and analysis. Additionally, the modular approach allows the applicant to potentially resolve any deficiencies noted by FDA earlier in the review process than would occur with a traditional PMA or HDE application.
This modular review approach is recommended for products that are in early stages of the clinical study. This modular review approach is not appropriate when the applicant is very close to submitting an original PMA or HDE or when the device design is in a state of flux or likely to change. FDA staff consider multiple scientific and regulatory approaches, consistent with least burdensome requirements and principles,4 to streamline regulatory processes during the review of modular PMAs and HDEs.
III. Scope 🔗
The modular review program is an alternative to the preparation, submission, and evaluation of traditional PMAs and HDEs. Because FDA believes that PMA and HDE supplements would rarely be appropriate for modular review, the scope of this document is limited to applicants pursuing approval of original PMAs and HDEs.
IV. Definitions 🔗
Shell is an outline and description of the contents for each of the modules that will comprise the PMA or HDE. This document also lays out the timeline and submission plan of the individual sections or “modules.”
Modular PMA or HDE is a compilation of sections or “modules” submitted following a pre-determined and an agreed-upon timeline that, together, become a complete PMA or HDE application after the final module is received.
Module is a discrete section of the PMA or HDE that can be submitted and reviewed independently. A module is a set of elements, tests, information, etc., that addresses a select discipline or group of disciplines (e.g., biocompatibility, non-clinical, and engineering bench testing) of the device application. These disciplines can be grouped in each module at the discretion of the applicant with review team concurrence, with the exception of manufacturing and Bioresearch Monitoring (BIMO) information. The manufacturing and BIMO information should be submitted in separate, standalone modules unless they are included as part of the final clinical module.
Module Amendment is information an applicant submits to the FDA to modify a module that is under review or closed. For example, an applicant’s response to a deficiency letter or updated contact information can be submitted as an amendment to a module.
V. User Fee Considerations for Modular Review 🔗
As discussed in the guidance “User Fees and Refunds for Premarket Approval Applications and Device Biologics License Applications,” the fee for a modular PMA with a valid eCopy is due upon submission of the first module. If FDA receives the first module of a PMA prior to payment, it will not be accepted for review until we receive payment5 and FDA will notify the applicant of this action by email.6 FDA begins its review when the Office of Financial Management notifies CDRH or CBER that payment has been received. For additional information, see FDA’s guidance, “eCopy Program for Medical Device Submissions.” Lastly, user fee refunds for modular PMAs are handled differently than traditional PMAs or for original PMAs that were converted from modular PMAs.7
Unlike traditional PMAs and modular PMAs, modular HDEs, like traditional HDEs, are not subject to user fees.8
VI. Industry Instructions for Submitting a Modular PMA or HDE 🔗
A. Contact Review Group 🔗
Appendix I includes a flowchart of the steps ordinarily involved in modular review. The first step should be contacting the Assistant Director/Branch Chief within the appropriate review division in CDRH or the appropriate Review Office Regulatory Project Management Director in CBER via email to indicate your intention to submit a modular PMA or HDE. For assistance in identifying the appropriate review division and team, contact the Division of Industry and Consumer Education (DICE)9 for CDRH-regulated devices or CBER’s Office of Communication, Outreach, and Development (OCOD)10 for CBER-regulated devices. FDA believes early interaction with the appropriate division/team and continued communication will optimize the opportunity for success of the modular PMA or HDE.
B. PMA or HDE Shell 🔗
(1) Content of a Shell 🔗
When you contact the appropriate Assistant Director/Branch Chief or Review Office Regulatory Project Management Director (through email), you should include a draft of the proposed shell. FDA recommends that applicants use the sample shell (Appendix II - Sample Shell) as a model when designing shell proposals. In your proposal, you should describe the contents of each module in sufficient detail to provide FDA with a complete understanding of the modules you plan to submit. The shell proposal should address all elements required under 21 CFR 814.20 for PMAs and 21 CFR 814.104 for HDEs, as well as the projected submission date of each module.
If the clinical trial period will be lengthy or the product development timeline is long, you should carefully consider your schedule for submitting modules when developing your shell. Premature submission of modules (i.e., prior to finalizing your device design) could result in changes to the device that require you to submit additional data and FDA to re-evaluate closed modules.
FDA may not grant a proposal for a shell if the planned timing for modular submissions is inappropriate. For example, if you are within 6 months or less of submitting the final module, FDA believes modular review may not be appropriate because it may not allow sufficient time for FDA to complete its review of all the modules before receiving the final module.
In addition, applicants should not plan to submit modules in close succession. The schedule for submitting modules should allow enough time for the review division to complete review of one module before the next module is received (i.e., at minimum 90 days). Submitting modules for concurrent review undermines the purpose of the modular review program because the Agency cannot review multiple modules on an abbreviated cycle. If you intend to submit several modules at the same time or in close succession, FDA recommends that you submit a traditional PMA or HDE instead.
The exception to this submission plan may be the manufacturing module. If resources permit, it may be possible for this module to be reviewed concurrently with other modules. This timing should be discussed with the review team during the shell review.
(2) Informal Review of the Proposed Shell 🔗
The proposed modular shell should be discussed and agreed upon with the FDA review team interactively before it is formally submitted to the Document Control Center (DCC). Immediately upon receipt of an informal request for a PMA or HDE modular review, the Assistant Director/Branch Chief or Review Office Regulatory Project Management Director assigns the informal request to a review staff member. This individual is responsible for communicating any FDA recommended changes and reaching agreement on the shell.
a.Determination that PMA or HDE Review is Appropriate for the Device
The first step of the informal review is confirming that PMA or HDE review is appropriate for the device. The assigned reviewer answers a set of preliminary questions in the PMA Acceptance Review Checklist, as found in the FDA guidance, “Acceptance and Filling Reviews for Premarket Approval Applications (PMAs)” or the HDE Filing Review Checklist, as found in the FDA guidance, “Humanitarian Device Exemption (HDE) Program,” respectively to determine PMA or HDE eligibility.11 Depending upon the answers to these preliminary questions, regulation as a class III device may not be appropriate. If the responses to the preliminary questions indicate that review of the modular proposal should not continue, the reviewer notifies the applicant using the procedures identified in the guidance documents referenced above.
b.Review of Proposed Shell Contents
After confirming that a PMA or HDE is the appropriate regulatory pathway, the reviewer assesses the contents of the proposed shell and communicates any recommended changes. If agreement cannot be reached with the assigned reviewer within 30 days, contact the appropriate Team and/or Division management.
(3) Formal Submission of the Finalized Shell 🔗
After you reach agreement with FDA on the proposed shell, we recommend that you follow the steps below to submit your finalized shell.
If you are submitting to CDRH, we recommend that you submit the finalized shell electronically via the Customer Collaboration Portal (“CDRH Portal”) as described on the Send and Track Medical Device Premarket Subsmissions Online: CDRH Portal webpage. Once submitted via the CDRH Portal, the shell will be received by the CDRH Document Control Center (DCC). Alternatively, the finalized shell may be mailed to the CDRH DCC. The current mailing address for CDRH’s DCC is provided on the eCopy Medical Device Submission webpage.
If you are submitting to CBER, we recommend that the finalized shell be submitted electronically through the FDA Electronic Submission Gateway. Alternatively, it can be submitted through the CBER submission email inbox (150MB max) at CBERDCC_eMailSub@fda.hhs.gov, or via mail to the CBER DCC. Additional information on the FDA Electronic Submission Gateway and the current mailing address for the CBER DCC can be found on the Regulatory Submissions in Electronic Format for CBER-Regulated Products webpage.
To facilitate the log-in process, you should prominently identify the name of the review staff member and the respective Office and Division with whom you have been interacting in your cover letter. It may also be helpful to include in your submission a PDF copy of the email correspondence where they provided concurrence on your proposed shell. After the FDA receives your submission and assigns a unique document control number (shell number), the review staff will confirm that the finalized shell is consistent with the agreed upon proposal and issue an acceptance correspondence (e.g., email) within 15 days from receipt. You can then submit modules for review, according to the agreed upon schedule.
(4) Changes to the Accepted Shell 🔗
If you need to make changes to the shell after it has been formally submitted and accepted (e.g., device design modifications are made that necessitate additional or different testing or the number of necessary modules increases or decreases), you should notify the review team via email and provide an explanation of why the changes are necessary. After informally reviewing these changes, FDA may wish to discuss them with you. Changes to the timing or the order of the modules may be made after review team concurrence is received via email. For significant changes to the content, title of each module, or number of modules, you should formally submit the revised shell as an amendment as you did for the finalized shell.
C. PMA and HDE Modules 🔗
(1) Submission of Each Module 🔗
When submitting a module, you should clearly identify the submission as a module and reference the previously assigned shell number and module title in the cover letter. FDA will assign module numbers in sequential order as modules are received. As outlined in Appendix II Sample Shell, you should include the following in every module:
- cover letter, addressed to the review staff member, Office, and Division who reviewed the shell;
- table of contents;
- executive summary of the testing and results included in the module;
- a copy of or reference to FDA’s Humanitarian Use Device (HUD) designation letter (for HDE modules);
- a brief device description and principles of operation; and
- bibliography (with references only to articles relevant to that module).
Duplication of some information between modules (e.g., device description) is necessary to allow the review staff assigned to that module to review it in an efficient manner.
(2) Time Frame for Reviewing a Module 🔗
FDA’s objective is to complete the review of each module and issue a deficiency letter or an acceptance correspondence within 90 days of receipt of the module. The modular review is intended to be interactive in nature, within reason. The review team may request minor clarification or additional information from the applicant interactively, as needed, during the review of each module. Deficiency letters notify the applicant of outstanding issues that need clarification or additional information before completing the review of the module. An acceptance correspondence notifies the applicant that all issues within the module have been resolved. Each module can continue to be submitted within its projected submission date identified in the shell regardless of prior modules’ review status in the series (e.g., awaiting response to deficiency letter from a previous module). Changes to the projected submission date for any module should be communicated to the FDA review team via email and agreed upon, as noted in Section VI.B.(4).
FDA intends to review amendments responding to a module deficient letter under a 90-day review clock. When FDA issues a module deficient letter, we stop the FDA review clock for the module and place the module on hold. When FDA receives a complete response to the module deficient letter, we consider it an amendment responding to a module deficient letter, the review clock is reset, and the amendment is reviewed within 90 days. Submitting an unsolicited major amendment, as described in 21 CFR 814.37(c)(1) for PMAs and 21 CFR 814.106 for HDEs, at any time during the review of the PMA or HDE may result in a delayed response from FDA.
(3) Incomplete Modules 🔗
The full benefit of modular review to both FDA and the applicant cannot be realized if module submissions are incomplete. Therefore, you should complete all testing needed to support a specific module before submitting it to FDA for review. In the event of an incomplete module submission, FDA intends on issuing a deficiency letter to the applicant stating that the review may not proceed until we receive the missing information. FDA intends to review each module within a 90-day period, beginning upon submission of an amendment containing the missing information.
(4) Reopening a Closed Module 🔗
If you modify your device after FDA determines a module is acceptable, you should contact the appropriate review staff to discuss and informally review the modification and any testing needed to support it. If review staff believe the modification is a major change, you should submit an amendment to each of the affected modules with a revised executive summary, change description, list of testing repeated, and data generated to support the modification. This amendment will reopen the relevant modules. As described above, FDA intends to issue a deficiency letter or an acceptance correspondence within 90 days of receipt of the amendment. If there are any changes to policies and regulations applicable to your device that occur after your module submission, FDA recommends that you contact review staff to discuss if your module should be updated and, if so, how these updates should be submitted. Updates that are not submitted as an amendment should be included in the final module.
(5) Submission of the Final Module 🔗
An applicant’s submission of the final module (i.e., final clinical data, proposed labeling, and Summary of Safety and Effectiveness (SSED) for PMAs or Summary of Safety and Probable Benefit (SSPB) for HDEs), plus the incorporation by reference of previously submitted modules, will complete the modular application. You should clearly identify the final module submission as the “COMPLETED MODULAR PMA or HDE” in the cover letter. You should specifically reference (by shell and module titles) the modules that have been accepted by the FDA and identify any modules with outstanding deficiencies. For the PMA or HDE to be complete, you should provide responses to all outstanding deficiencies in the final module.
Upon receipt of the final module, FDA converts the completed modular application into an original PMA or HDE and assigns it a PMA or HDE number.
(6) Acceptance and/or Filing after Conversion to Original PMA or HDE 🔗
Following the conversion of the completed modular application, review will proceed as an original PMA or HDE where FDA makes its acceptance and/or filing decision. This decision is based on whether the final module includes all the information necessary to complete the application as required by 21 CFR 814.20 for PMAs and 21 CFR 814.104 for HDEs.
For a PMA, the FDA guidance “Acceptance and Filling Reviews for Premarket Approval Application (PMAs)” describes the kind of information required under 21 CFR 814.20 that needs to be submitted before FDA can file the application. FDA plans to use the information described in this guidance in making acceptance and filing determinations. As for a traditional PMA, FDA generally issues an acceptance or refuse to accept decision within 15 days of receipt of the final module and, if accepted, a filing or non-filing decision within 45 days of receipt of the final module. If FDA decides to file the PMA, the filing date is the date that the application became complete, typically the receipt date of the final module. The 180-day "PMA clock" under 21 CFR 814.40 also begins on that date. If the Agency decides not to accept the PMA, FDA will issue a correspondence noting the missing items. If the Agency decides not to file the PMA, FDA will issue a non-filing decision to the PMA applicant.
For an HDE, the FDA guidance, “Humanitarian Device Exemption (HDE) Program” describes the kind of information required under 21 CFR 814.104 that needs to be submitted before the FDA can file an HDE. For an HDE, FDA will issue a filing or non-filing decision within 30 days of receipt of the final module.12 If FDA decides to file the HDE, the filing date is the date that the application became complete, typically the receipt date of the final module. The 75-day “HDE clock” under 21 CFR 814.116 also begins on that date. If the Agency decides not to file the HDE, FDA will issue a non-filing decision to the HDE applicant.
Appendix I – Modular PMA or HDE Flowchart 🔗
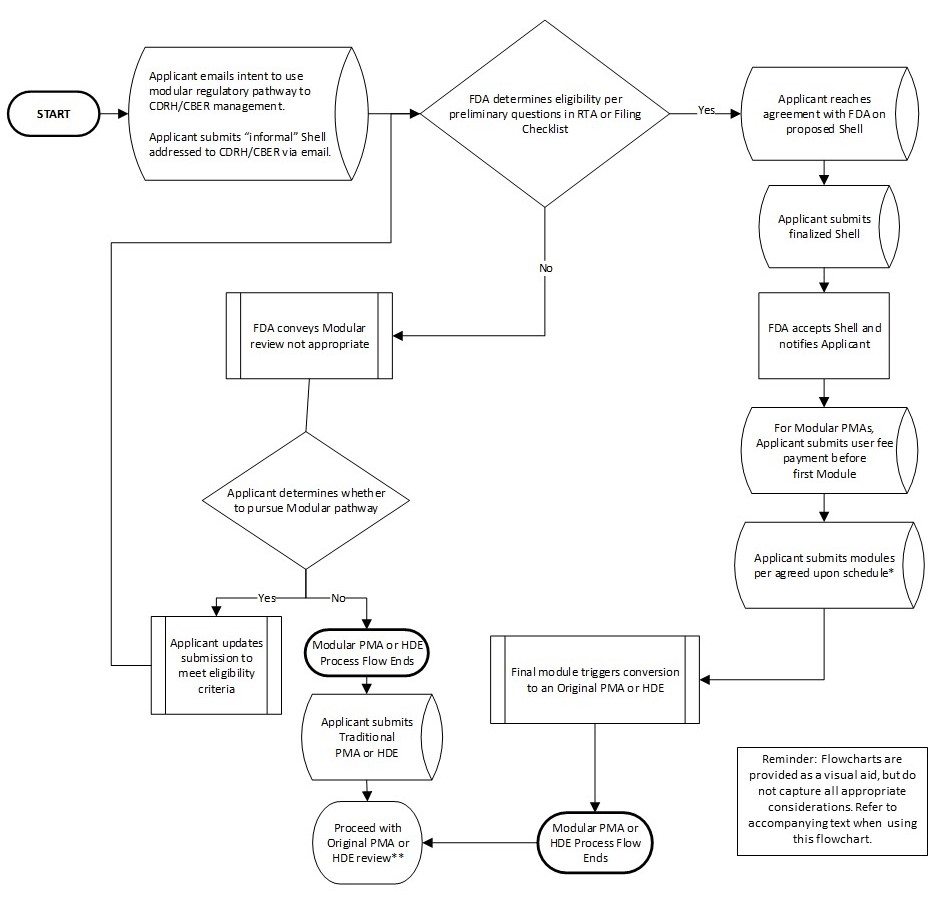
- Each module is subject to a 90-day review clock. You should factor in this timing when determining when to submit each module. The exception may be the manufacturing module, which, if resources permit, can be submitted irrespective of when the other modules are submitted.
- * Original PMA or HDE review begins with RTA and RTF review phases before beginning with substantive review, as done with traditional PMAs or HDEs.
Appendix II – Sample Shell 🔗
Company / Device Name
| Title | Contents | Projected Date of Submission |
|---|---|---|
| Module Title (e.g., Physico-chemical, biocompatibility/toxicity, animal/biological) | Table of Contents for [Title of Module, e.g., Physico-chemical] Module Executive Summary* Copy of or reference to HUD designation letter (for HDE modules) Device Description and Principles of Operation Declaration of Conformance to Standards for Module Bibliography/References for Module Non-clinical Laboratory Studies, for example: • Physico-Chemical Testing • Biocompatibility/Toxicity Testing • Animal/Biological Testing |
|
| Module Title (e.g., Engineering/bench, sterilization/shelf-life/packaging, software) | Table of Contents for [Title of Module, e.g., Engineering] Module Executive Summary* Copy of or reference to HUD designation letter (for HDE modules) Device Description and Principles of Operation Declaration of Conformance to Standards for Module Bibliography/References for Module Non-clinical Laboratory Studies, for example: • Engineering/Bench Testing • Sterilization, Shelf Life & Packaging Information (Pertinent information should also be provided in the manufacturing section) • Software Validation and Verification Information |
|
| Module Title (e.g., Manufacturing)** | Table of Contents for [Title of Module, e.g., Manufacturing] Module Executive Summary* Copy of or reference to HUD designation letter (for HDE modules) Device Description and Principles of Operation Manufacturing Information • Refer to “Quality System Information for Certain Premarket Application Reviews; Guidance for Industry and FDA Staff” |
|
| Final PMA or HDE Module | Table of Contents for entire PMA or HDE, including all modules Copy of or reference to HUD designation letter (for HDE modules) SSED or SSPB (i.e., compilation of executive summaries) Clinical Data (including Protocols, Results, and Analyses) Financial Disclosure Information Proposed Labeling: • Physician Instructions • Patient Instructions • Operation Manuals Post-marketing Plan (e.g., proposed long-term follow up studies, if appropriate) Bibliography/References for the Final Module |
- Executive Summary should contain a summary of the testing and results provided in the module.
- * Manufacturing module should be submitted as a separate, standalone module and not grouped with other disciplines.
Appendix III – Frequently Asked Questions (FAQ) 🔗
Contact the Assistant Director/Branch Chief or Review Office Regulatory Project Management Director within the appropriate review division in CDRH or CBER for any specific questions not covered in this FAQ.
1. What is the “sample shell”? 🔗
An example of a modular shell is provided in Appendix II of this guidance document. FDA recommends that you follow this model whenever possible. Ideally, there should be no more than 3-4 modules. However, we understand that there may be instances when the recommended model is not suitable and should be modified. The review staff will work with you to establish a shell that is acceptable to both parties during the shell review.
2. What should I consider when deciding whether to submit a Modular PMA or HDE? 🔗
The intent of the modular review approach is to provide a mechanism by which applicants can submit non-clinical data and manufacturing information for review while still collecting, compiling, and analyzing the clinical data using the final device design.
FDA recommends that you carefully consider the timeline for the development of your product and your commitment to pursuing FDA approval when deciding whether to submit a modular PMA or HDE. FDA may not accept a PMA or HDE shell proposal if the timing for submitting modules is inappropriate. For example, if you are within 6 months or less of submitting the final module, FDA believes modular review may not be appropriate. Submitting a shell proposal within 6 months or less of submitting the final module does not allow sufficient time for FDA to complete its review of all the modules before receiving the final module.
If the clinical trial is lengthy or the product development timeline is long, you should carefully consider your schedule for submitting modules when developing the shell. Premature submission of modules could result in changes that require re-evaluation of closed modules.
3. What is an appropriate length of time between submission of modules? 🔗
You should provide the approximate timing of the submission of each module to FDA in the shell. Applicants who choose to participate in the modular review program should submit modules at intervals (i.e., at minimum 90 days) that will allow the Agency enough time to review the module, provide feedback, and close out the module prior to the arrival of the next module. The exception to this may be the manufacturing module, which can be reviewed concurrently with other modules if resources permit. Such timing should be discussed with the review team during the shell review.
4. What should I include with each module? 🔗
You should include the following in each module:
- cover letter, addressed to the review staff, Office, and Division who reviewed the shell;
- table of contents;
- executive summary of the testing and results included in the module;
- a copy of or reference to FDA’s HUD designation letter (for HDE modules);
- a brief device description and principles of operation; and
- bibliography (with references only to articles relevant to that module).
In addition, you should submit complete information for each module (i.e., all testing should be finished, and the results provided), which will enable FDA to review the data and make a decision. Duplication of some information (e.g., device description) may be necessary to allow the review staff assigned to that module to review it in an efficient manner. If the module is incomplete, FDA intends on issuing a deficiency letter for the module under review.
5. Does the Modular PMA or HDE include an RTA and/or RTF phase? 🔗
Modular PMAs and HDEs do not have RTA or RTF reviews. There is a preliminary review conducted during informal review of the proposed shell to determine whether a Modular PMA or HDE is an appropriate regulatory pathway. However, upon receipt of the final module, FDA converts the completed modular application into an original PMA or HDE. At that time, review proceeds with RTA and/or RTF review phases before beginning substantive review, as done with traditional PMAs and HDEs.
6. Does the clinical module need to be submitted last? 🔗
The general intent of this program is to enable applicants to address issues, if present, with the non-clinical data in each of the modules while the applicant simultaneously prepares and collects clinical data using the final device design for the clinical module. For that reason, it is highly recommended that the clinical module be the final module. BIMO information can be submitted as its own module or as part of the clinical module. If you do not plan to submit the clinical module as the final module, we recommend that you discuss your modular plan with FDA to determine if it is appropriate, prior to submitting the modular shell proposal.
7. Is interactive communication utilized during modular review? 🔗
Modular reviews are intended to be interactive in nature. The review team may ask for clarification or additional information interactively via email, phone call, or informal teleconference during each module review. However, we understand that certain information may not be readily available at the time of our request. In this case, FDA may make these requests in a deficiency letter that can be addressed as an amendment to the current module or at the time of final module submission.
In CDRH, a reviewer from an Office of Health Technology (OHT) will be assigned as the primary point of contact for each module. In CBER, both the Office of Compliance and Biologics Quality and the Product Review Office review modules; therefore, you should discuss points of contact with the appropriate regulatory project management branch in CBER.
8. Can I request an extension of time to address all deficiencies identified in a deficiency letter? 🔗
There are no formal extension requests for amendments. If you intend to address the deficiencies prior to the submission of your final module, you should submit the information in the form of an amendment to the relevant module. If deficiencies are not addressed prior to submission of your final module, you should provide responses to the deficiencies in the final module.
9. What happens to the outstanding modules when I submit my final module? 🔗
You should address any outstanding deficiencies from previously submitted modules when you submit the final module. Once you submit the final module, FDA considers the shell and its modules closed and converts the completed modular application into an original PMA or HDE. All information from current and prior modules will be factored into the review of the original PMA or HDE. FDA will then make its acceptance and filing decisions for a PMA or a filing decision only for an HDE before proceeding with substantive review.
10. When do I have to pay the user fee for a Modular PMA? 🔗
The fee for a modular PMA is due upon submission of the first module; see FDA guidance “User Fees and Refunds for Premarket Approval Applications and Device Biologics License Applications.” If FDA receives the first module of a PMA prior to payment, it will be not accepted for review until we receive payment13 and FDA will notify the applicant of this action by email.14 The 90-day review clock for the first module starts on the date that FDA’s Office of Financial Management notifies CDRH or CBER that payment has been received and a valid eCopy has been provided.
Footnotes 🔗
-
Refer to website, PMA Guidance Documents | FDA, for links to PMA guidance documents. ↩
-
See FDA’s guidance, “Humanitarian Device Exemption (HDE) Program.” ↩
-
Section 515(c) of the FD&C Act, as amended by Section 209 of MDUFMA. ↩
-
See FDA’s guidance, “The Least Burdensome Provisions: Concept and Principles,” and sections 513(a)(3)(D)(ii), 513(a)(3)(D)(iii), 515(c)(5)(A), 515(c)(5)(B), and 515(c)(5)(C) of the FD&C Act. ↩
-
See section 515(c)(4)(A) of the FD&C Act. ↩
-
Section 738(f)(1) of the FD&C Act authorizes FDA to not accept an application until payment is received. ↩
-
See FDA’s guidance “User Fees and Refunds for Premarket Approval Applications and Device Biologics License Applications” and section 738(a)(2)(D) of the FD&C Act. ↩
-
See FDA’s guidance, “Humanitarian Device Exemption (HDE) Program” and section 738(a)(2)(B)(i) of the FD&C Act. ↩
-
Refer to the website Contact Us – Division of Industry and Consumer Education (DICE) for contact options. ↩
-
The OCOD can be contacted at 1-800-835-4709 or 240 402-8010, or by email at ocod@fda.hhs.gov. ↩
-
These questions are found under the “Preliminary Questions” section in both checklists; questions 1-7 in the PMA Acceptance Review Checklist and questions 1-5 in the HDE Filing Review Checklist. FDA does not review the entire checklist during this step. ↩
-
21 CFR 814.112(a) ↩
-
See section 515(c)(4)(A) of the FD&C Act. ↩
-
Section 738(f)(1) of the FD&C Act authorizes FDA to not accept an application until payment is received. ↩

