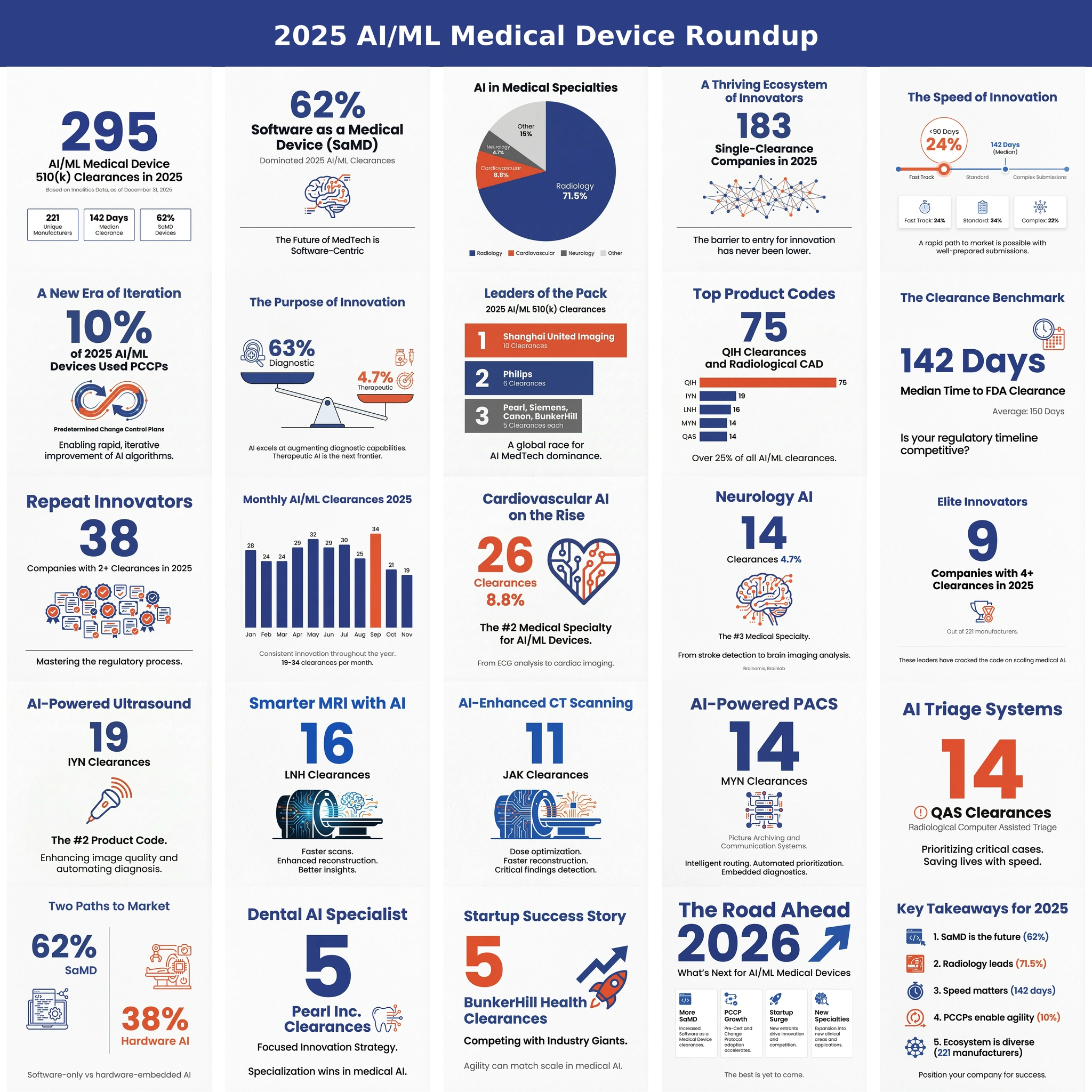
Why You Need a Breakthrough Device Designation (BDD) for Your AI/ML SaMD? 🔗

The FDA’s Breakthrough Device Designation can be viewed as a form of intellectual capital because it represents institutional validation of innovation that adds strategic value beyond the physical device. Although it does not provide legal exclusivity like a patent, the designation signals that the device addresses an unmet clinical need and offers meaningful advantages over existing options. This regulatory recognition embeds credibility, specialized knowledge, and regulatory insight within the organization, functioning as an intangible asset that strengthens the firm’s innovative standing.
The FDA’s Breakthrough Device Designation can be seen as a valuable intangible asset because it shows official recognition that a device is innovative and clinically important. While it does not provide patent-like protection, it adds value to an organization by:
- Confirming the device addresses an unmet medical need
- Signaling meaningful improvement over existing options
- Building credibility with regulators, investors, and partners
- Capturing regulatory knowledge and experience within the organization
- Strengthening the company’s reputation for innovation
Like patents, Breakthrough Device Designation is hard for competitors to copy quickly, which makes it a valuable form of intellectual capital. It is earned through real innovation, clinical importance, and close work with the FDA—not just spending money. The experience and regulatory insight gained through this process carry over to future products. As a result, the designation acts as a lasting intangible asset that complements patents and strengthens a company’s long-term competitive position.
Compared to patents, Breakthrough Device Designation is a more accessible form of intellectual value for innovators. While patents are often expensive, slow, and uncertain, the FDA designation offers several practical advantages:
- No application or maintenance fees
- A much faster decision process (often within 60 days)
- No multi-year legal process or ongoing prosecution costs
- Clear recognition tied directly to clinical importance and innovation
These features make Breakthrough Device Designation especially valuable for early-stage or resource-limited companies seeking meaningful intangible value without the burden of patent costs.
Even though it is fast and low cost, Breakthrough Device Designation can offer strategic value comparable to—or greater than—a patent. Patents protect technical ideas but do not reduce regulatory risk or speed up FDA approval. In contrast, the designation provides real regulatory benefits, such as earlier FDA access, faster feedback, and more flexible clinical requirements. These advantages can shorten development timelines, save money, and increase the impact of the underlying technology. For investors and partners, gaining this value quickly and at no direct cost makes the designation a high-return complement to traditional intellectual property.

The fact that Breakthrough Device Designation is free does not make it easy to obtain. It is awarded only to devices that clearly show strong innovation and address an unmet medical need. Unlike patents, which can be pursued broadly, this designation is granted selectively based on FDA judgment. Its combination of zero cost, fast review, and strong credibility makes it a rare and powerful form of intellectual value that complements patents and offers a quicker, leaner path to competitive advantage in medical devices.
What Breakthrough Device Designation Is: 🔗

In the same way that a reservation at an exclusive restaurant does not change the quality of the food but guarantees access, attention, and timing, Breakthrough Device Designation secures privileged access to the FDA’s regulatory expertise. Instead of waiting in line, sponsors are seated promptly with decision-makers who understand the urgency and promise of the technology. This “reservation” allows for iterative feedback, flexible clinical evidence strategies, and faster resolution of issues that might otherwise delay development. As a result, the designation transforms the regulatory pathway from a crowded, uncertain process into a curated, high-touch experience—one that acknowledges the device’s potential impact and helps ensure it reaches patients as efficiently as possible.
Why Companies Fail to Obtain Breakthrough Device Designations? 🔗
Companies often overcomplicate the breakthrough device designation because they are not focusing on what the FDA cares about. Rather, they think of it more like a research paper or an investor pitch. This is the incorrect mindset. You need to convince the regulator that the fast tracking the device is in the best interest of the American public. It is not just a technical write-up or paperwork exercise—it is a persuasive case to the FDA. A successful request must:
- Clearly show the device addresses a serious unmet medical need
- Explain how it meaningfully improves on existing options
- Make a convincing, evidence-based argument to expert reviewers
Treating the process casually can weaken the case and lead to missed opportunities to demonstrate real clinical value and differentiation.
An experienced regulatory professional plays a role similar to a brilliant lawyer arguing a case before the Supreme Court. Just as a great lawyer does not merely recite facts but frames them within precedent, logic, and consequence, a seasoned regulatory expert understands how the FDA evaluates evidence, language, and risk. They know which data points matter most, how to acknowledge limitations without weakening the argument, and how to align the narrative with the agency’s statutory criteria. Without this level of advocacy, even a genuinely innovative device can fail to secure designation because its story is not told in a way that resonates with regulators.
Companies also underestimate the effort because the strongest case for Breakthrough Device Designation is rarely obvious or purely technical. Success often depends on nuance—how standard of care is defined, which alternatives are compared, and what truly counts as a meaningful clinical improvement. Experienced regulatory professionals anticipate FDA concerns and address them upfront. This level of strategic judgment is hard to replicate without deep regulatory experience.
Finally, companies may also overlook that a Breakthrough Device Designation request shapes the entire future relationship with the FDA. A strong submission does more than seek approval—it sets expectations. It:
- Establishes credibility and trust with FDA reviewers
- Shows clear, logical reasoning backed by solid evidence
- Demonstrates seriousness, judgment, and regulatory maturity
With experienced regulatory guidance, the request becomes not just a bid for designation, but a signal that the company understands the stakes and can operate at a high level throughout the regulatory process.
Tap into the Best Kept Secret of the Breakthrough Device Designation. 🔗
The FDA's Total Product Life Cycle Advisory Program (TAP) is one of the most strategic—yet underutilized—benefits of Breakthrough Device Designation. While most companies focus on faster review times, TAP delivers something more valuable: direct access to FDA leadership and expertise throughout the entire product lifecycle. This includes reimbursement and direct introductions to CMS, payers, and medical associations. There is nothing that can open more doors than an introduction from an fda.gov email. It transforms the agency from a regulatory hurdle into a strategic advisor, providing coordinated input from senior reviewers across clinical, regulatory, and statistical domains.

TAP delivers tangible business value:
- De-risk pivotal study design before committing capital
- Accelerate regulatory alignment on novel or first-in-class technologies
- Reduce costly late-stage pivots and delays
- Gain predictability that investors and board members value
- Get a better idea of pathway to revenue after FDA clearance
Why Breakthrough Device Designation Matters for Your Business: 🔗
1. Unlock Funding and Investment 🔗
Investors view BDD as a de-risking milestone because it signals the FDA agrees your device targets a serious condition and may be meaningfully better than current options.
2. Accelerate Your FDA Review 🔗
BDD provides mechanisms that change pace and responsiveness:
- Priority review for marketing submissions under the Breakthrough Devices Program
- Sprint discussions as a structured option for focused questions and faster alignment
- Senior management engagement as part of the program's "review team support" model
There's also empirical work suggesting breakthrough-designated therapeutic devices are often reviewed within target timeframes, though the evidence base and outcomes can be mixed.
Bottom line: you're more likely to avoid the "submit, wait, surprise objection, redesign" loop.
3. Reduce Regulatory Uncertainty 🔗
This is the most under-appreciated benefit.
AI/ML SaMD teams lose time and money on questions like:
- What endpoints will FDA accept for CADx or CADp claims?
- Reader study vs standalone? What does "clinically meaningful" look like here?
- What's the right comparator and reference standard, especially in pathology?
- For CADt, what workflow and use environment assumptions are acceptable?
FDA describes the program as enabling more timely interaction and support during development and review.
That early alignment can be the difference between:
- One well-designed pivotal study, or
- Two to three iterations that burn runway.
4. Competitive and Market Advantage 🔗
Breakthrough Device Designation gives companies a strong competitive edge by signaling that the FDA sees the device as addressing a serious unmet need with real clinical promise. It reduces regulatory uncertainty, speeds development, and provides closer FDA support that competitors do not have. This allows companies to reach the market faster, use capital more efficiently, and establish leadership early. The designation also acts as a credibility signal that helps differentiate the device, strengthen partnerships and reimbursement discussions, and increase overall company value.
Frequently Asked Questions 🔗
Does my device need to be done for a BDD? 🔗
A device does not need to be finished to qualify for Breakthrough Device Designation. The program is meant to support promising technologies early, not completed products. The FDA looks at whether a device has the potential to meaningfully improve care for a serious condition, not whether it is fully built or market-ready. Early entry into the program lets the FDA guide key design and clinical decisions while there is still flexibility, which helps reduce risk, speed development, and increase the chances that important innovations reach patients.
What is the process for submitting a BDD? 🔗
A strong Breakthrough Device Designation request starts long before writing the application. It begins with an experienced regulatory professional who understands how the FDA thinks and works. This person helps:
- Confirm the device truly meets the program’s criteria
- Choose the right indication and comparison to existing care
- Clearly explain the unmet medical need in FDA-friendly terms
- Frame the submission as a persuasive argument, not a checklist
- Anticipate and address FDA concerns upfront
Equally important is genuine engagement with the technology and its clinical impact. A regulatory expert who cares about the innovation can turn complex details into a clear, credible story that resonates with FDA reviewers. This approach encourages productive dialogue, improves the chances of success, and sets the stage for a strong long-term relationship with the FDA.
Can I submit a Presub in parallel? 🔗
Yes, a Pre-Submission (Q-Sub) can be filed at the same time as a Breakthrough Device Designation request, and this is often a smart strategy. Doing both allows a company to seek Breakthrough recognition while also getting FDA feedback on specific regulatory, clinical, or technical questions. When coordinated well, the Breakthrough request focuses on the device’s overall value and unmet need, while the Pre-Sub covers the practical details. Together, they help companies align with the FDA faster and get more value from early engagement.
Can software-only devices qualify? 🔗
Yes, if they meet the serious-condition and "may be more effective" criteria.
Will a BDD force me to go down a De Novo pathway? 🔗
No.
Is BDD a clearance or approval? 🔗
No. You still need a 510(k), De Novo, or PMA and must meet the evidentiary standards.
Do we need prospective trials to apply for BDD? 🔗
Not necessarily. Many teams apply with retrospective or preliminary evidence, then use the program to align on pivotal expectations. FDA's process focus is accelerated interaction and review, not skipping evidence.
Can BDD be withdrawn? 🔗
FDA guidance describes circumstances where designation can be withdrawn, including if the device no longer meets eligibility or if material information was misstated or omitted.
When is the right moment to apply for BDD? 🔗
The right moment is when the device concept, intended use, and clinical value proposition are stable enough to tell a credible “may be more effective” story, but early enough that FDA input can still influence study design, endpoints, and development strategy. Typically, this is after initial feasibility or retrospective validation exists, but before pivotal trial design is locked. Too early feels speculative; too late forfeits much of BDD’s value.
What happens if we apply too early? 🔗
An early rejection does not poison future interactions or prevent reapplication. FDA decisions are evidence-based and contextual; if new data, refined claims, or clearer comparators emerge, a resubmission can be evaluated cleanly. The real downside of applying too early is opportunity cost—wasting a chance to frame the narrative persuasively—rather than reputational harm.
Can BDD meaningfully shorten time to submission, even if clearance still takes years? 🔗
Yes. While total calendar time to clearance may still be long, BDD compresses decision-making time. Study design converges faster, endpoint debates resolve earlier, evidence expectations are clarified sooner, and fewer late-stage surprises occur. These efficiencies often shave months—or more—off development even if the final review clock remains substantial.
Should BDD be part of our fundraising plan, or treated as purely regulatory? 🔗
BDD is both regulatory and strategic. At Seed and Series A, investors often view it as strong validation of unmet need and regulatory credibility. By Series B, it is seen as risk-reduction and execution leverage rather than novelty. Savvy investors understand that BDD does not guarantee clearance, but they value the signal and the access it provides.
How does BDD interact with enterprise partnership discussions? 🔗
BDD changes diligence conversations from “Is this plausible?” to “How fast and how big?” Strategics and health systems often interpret designation as evidence that the FDA sees real clinical relevance. It can reduce skepticism, accelerate technical diligence, and shift focus toward integration, scalability, and commercial strategy.
What level of model performance is “enough” for BDD? 🔗
FDA does not require superiority or non-inferiority in the statistical sense. What is required is a credible hypothesis supported by data that the device may be more effective for a serious condition. This can be contextual, task-specific, or population-specific and depends heavily on comparator choice and clinical framing.
How much clinical data is typically sufficient for a BDD application? 🔗
There is no fixed number. Some applications succeed with dozens of well-curated cases; others require hundreds or more. The sufficiency depends on claim scope, outcome type, risk profile, and whether the data convincingly supports the narrative. Quality, relevance, and framing often matter more than raw volume.
Can synthetic data or enrichment strategies support a BDD argument? 🔗
They can support—but not replace—real clinical data. Synthetic data may help demonstrate robustness, stress testing, or rare-event performance, while enrichment strategies can clarify value in high-risk subpopulations. FDA expects transparency, justification, and clear boundaries around what these methods do and do not prove.
How do we argue “meaningfully more effective” for workflow, triage, or productivity tools? 🔗
The argument must ultimately tie back to patient or system-level outcomes, even if indirectly. Reduced time to diagnosis, improved prioritization of critical cases, lower cognitive burden, or more consistent decision-making can qualify if the downstream clinical impact is plausible and well-articulated. FDA looks for credible causal chains, not just efficiency metrics.
Does FDA expect locked models at the BDD stage? 🔗
No. Iteration is expected and acceptable at the BDD stage. The FDA understands that early engagement is most valuable when designs are still evolving. What matters is clarity around the intended trajectory and guardrails for future changes, not finality.
Does BDD lock in our intended use or claims too early? 🔗
BDD does not lock claims, but it does establish a reference point. Companies retain flexibility to refine indications, claims, and evidence strategies post-designation. However, significant pivots may require re-framing discussions to ensure alignment with the original basis for designation.
Can BDD cover a platform or foundation model, or only a single indication? 🔗
BDD is typically granted for a specific indication and use case, not a broad platform. That said, a well-scoped request can be designed to support future expansion. Narrow, defensible claims tend to succeed more often than overly broad platform arguments.
What happens if our predicate strategy changes after BDD? 🔗
BDD is pathway-agnostic. A pivot from 510(k) to De Novo does not invalidate the designation. In fact, BDD can be especially valuable during such pivots by maintaining continuity of FDA engagement despite strategic shifts.
Can BDD coexist with PCCP planning for AI/ML updates? 🔗
Yes, and they can reinforce each other. BDD facilitates early dialogue about lifecycle management, while PCCPs provide a structured framework for post-market updates. Together, they signal seriousness about both innovation and control, provided the scope is realistic.
Does BDD affect postmarket expectations or scrutiny? 🔗
Potentially, yes—but not punitively. FDA may expect more thoughtful postmarket monitoring given the novelty and impact of the device. This is generally a tradeoff companies welcome in exchange for earlier access, clearer expectations, and stronger premarket alignment.
What do “sprint discussions” actually look like in practice? 🔗
Sprint discussions are focused, time-bound FDA interactions used to resolve specific questions quickly—such as endpoint selection, comparator choice, or study modifications. They are not open-ended brainstorming sessions and are most effective when well-prepared and narrowly scoped.
How much access to senior FDA reviewers do companies really get? 🔗
Access is real but case-dependent. Many BDD sponsors interact with senior reviewers earlier and more directly than in standard pathways. The quality of access often correlates with clarity of questions, preparedness, and the seriousness of the clinical issue being addressed.
Does BDD change how disagreements are resolved during review? 🔗
Yes. BDD can facilitate faster escalation, clearer internal FDA alignment, and continuity despite reviewer turnover. While it does not eliminate disagreement, it often shortens the path to resolution.
What kinds of questions should be saved for BDD interactions versus Pre-Sub? 🔗
Use Pre-Subs for structured, technical questions that require formal written feedback. Reserve BDD interactions for strategic alignment, tradeoffs, and forward-looking decisions. Avoid using BDD time for questions that could be answered through standard channels—reviewer goodwill is finite and should be used deliberately.
Closing Thoughts 🔗
If you're building AI/ML SaMD for serious conditions, stop asking whether BDD is "nice to have." The real question is: Do you want to derisk your pivotal strategy with FDA alignment—or burn capital on assumptions that may not hold?
BDD is one of the only levers that collapses regulatory uncertainty early, accelerates decision cycles, and simultaneously strengthens your story to investors and strategic partners.
Bottom line: Treat BDD like the high-stakes asset it is. Lock down your eligibility argument. Build a disciplined evidence narrative. Make your clinical impact case bulletproof. Then execute with a process built to win—not just check a box.
That's the entire playbook.
Next Steps 🔗
Want to put your AI/ML device's BDD on autopilot? We offer a turnkey Guaranteed Breakthrough Device Designation service.
Guaranteed deliverables:
- BDD eligibility assessment with FDA criteria mapping
- Complete BDD application package
- FDA eSTAR submission prepared and filed
- Strategic guidance on leveraging BDD for fundraising and partnerships
Revision History 🔗
| Date | Changes |
|---|---|
| 2025-12-11 | Initial Version |