Innolitics Tool
The MAUDE (Manufacturer and User Facility Device Experience) database houses the FDA’s MDR’s (Medical Device Reports). This database is the official repository of adverse events in the U.S.
It is useful to know when new MDR’s are added for your device product code. Without tooling, it would require one to manually search the database periodically. We wanted an automatic way of being notified when relevant MDRs were added.
In pursuit of our mission to accelerate progress in medical imaging, we created a free to use tool to send email alerts when new adverse events matching search criteria are reported. For example, you may want to know when a injury or death matching your product code is reported. Additionally, you may want to know when new events are reported against your predicate device to be a step ahead of an FDA warning letter. MAUDE-Alerts is a medical device market research tool that automates post-market analysis by sending alerts to you when new Medical Device Reports are reported.
Click here to jump right in and try the MAUDE-Alert tool.
The sign-up and log-in process for MAUDE-Alerts is password-less meaning you can start using the tool without having to remember yet another password.
MAUDE-Alerts is connected to the openFDA API. This API hosts all MAUDE Medical Device Reports and the database is updated weekly with new reports. The API contains Medical Device Reports from as far back as 1991, whereas the FDA’s MAUDE database search tool is limited to reports from the last 10 years.
The MAUDE-Alerts search is modeled after the search form on the MAUDE database search site. As shown in Figure 1 the same fields are provided to search for MAUDE reports, including a keyword option that searches all reports for a given word.
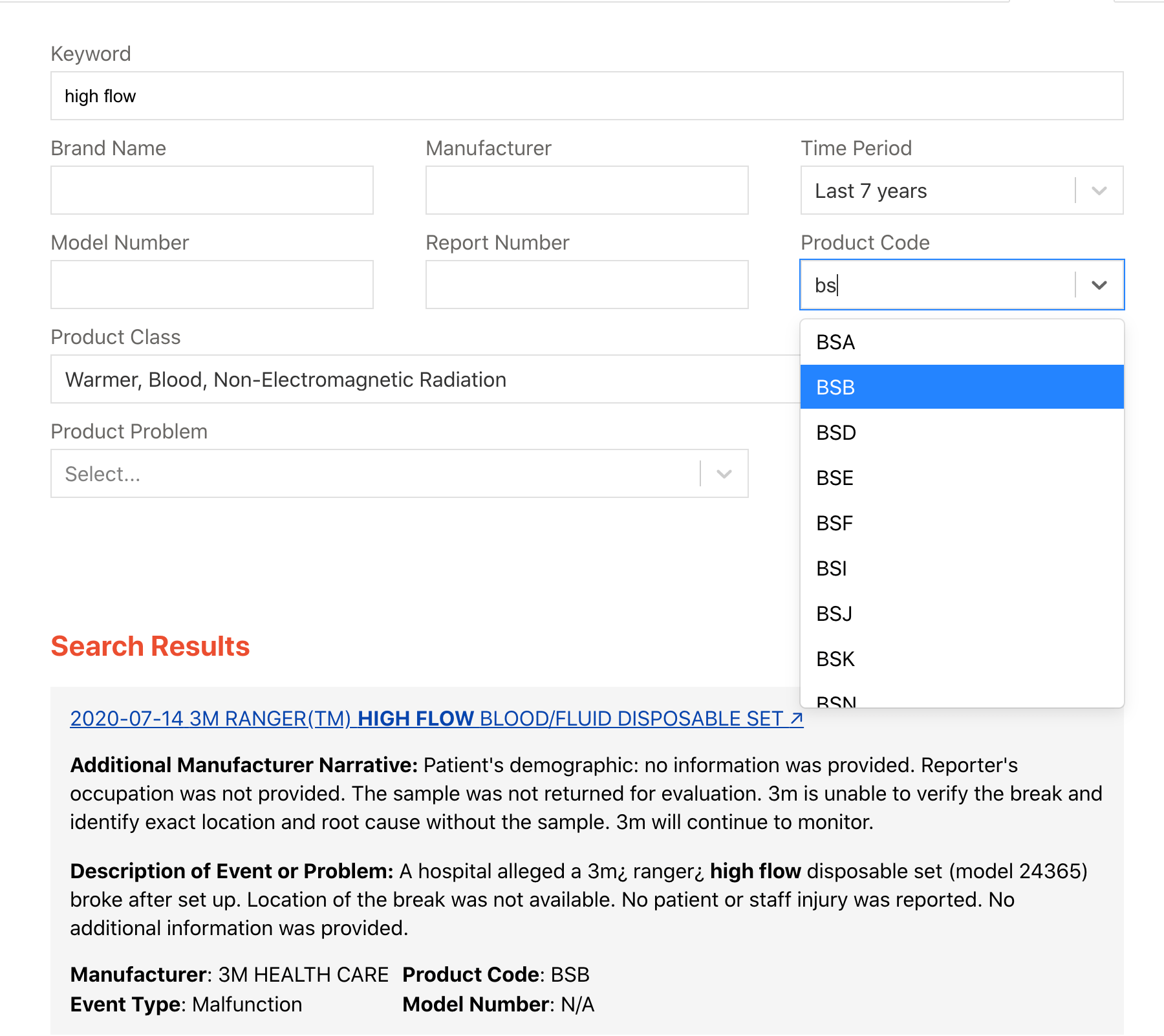
Our search form includes several usability enhancements, including auto-filled product code and product class, and Google-like search results design. Additionally it returns double the results returned by the FDA’s MAUDE site (1000 compared to 500).
The CSV export is located on the MAUDE-Alerts search results page and update emails. This allows market researchers to share and save data that is noteworthy or requires further analysis.
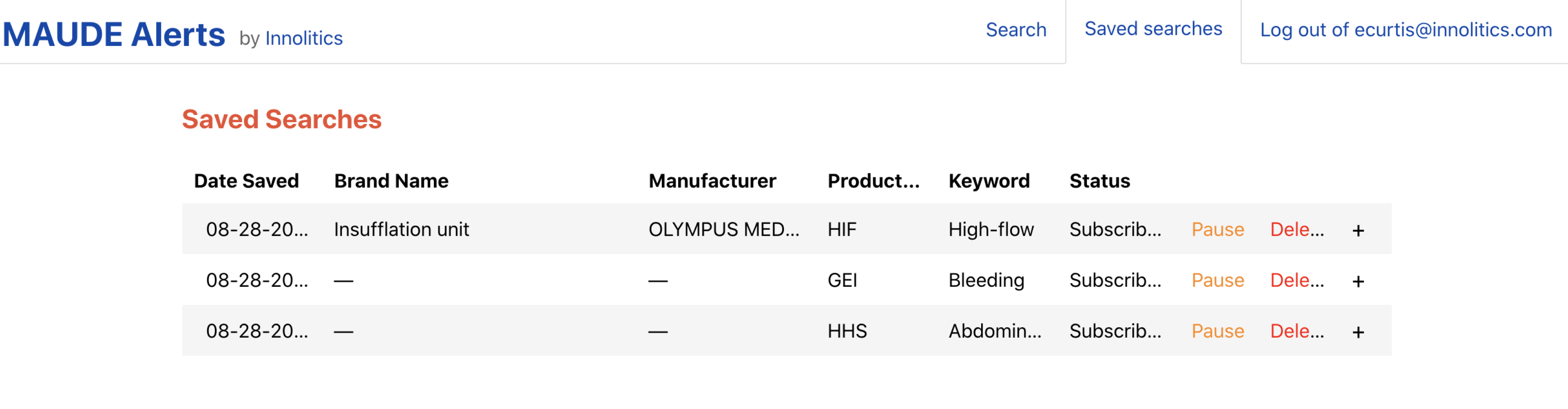
MAUDE-Alerts checks the MAUDE database weekly for any new reports matching your saved searches, Figure 2, and sends you an email report if we find anything. You can see an example of a notification email in Figure 3. We also give you the option to pause or delete alerts if necessary.
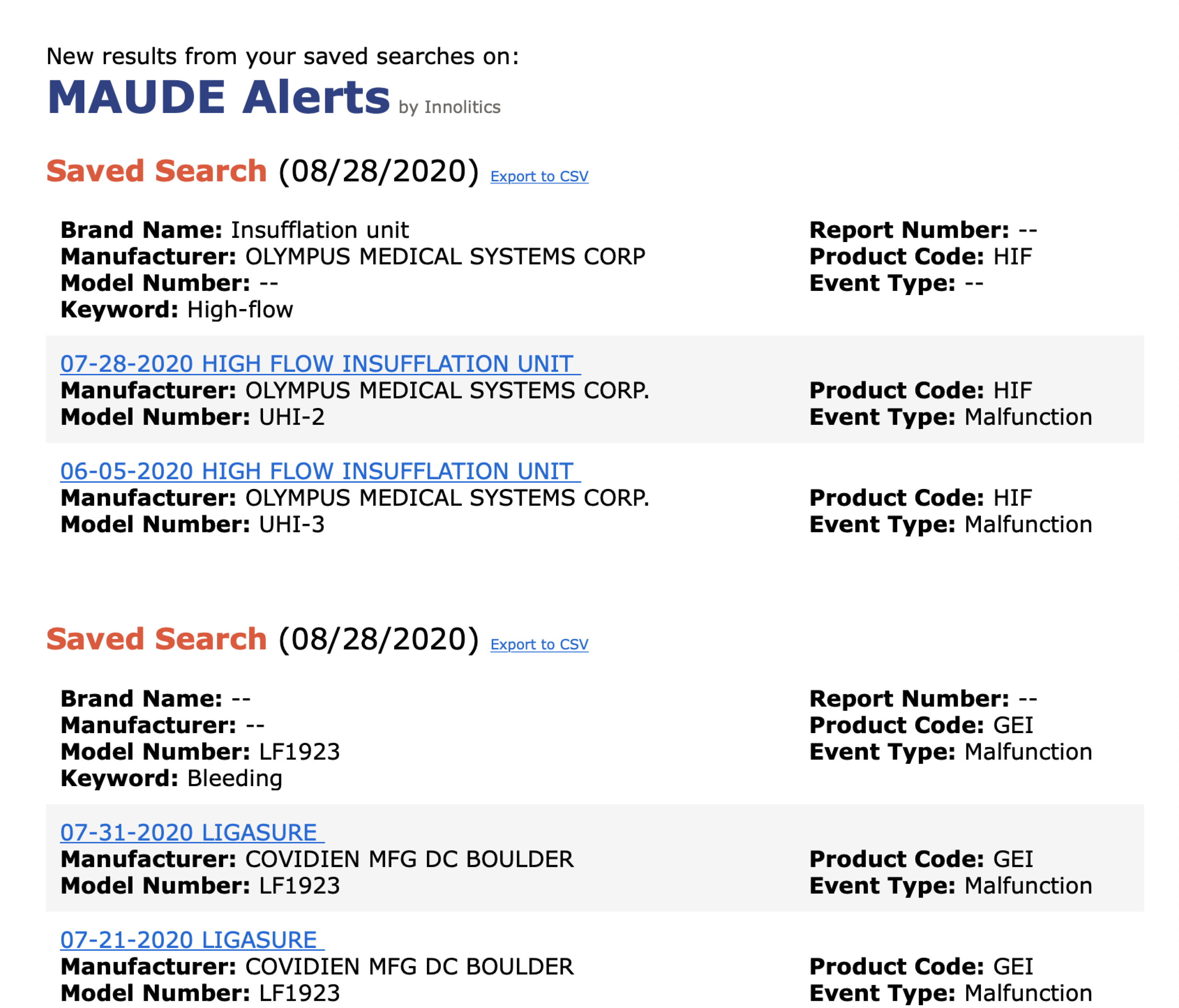
The MAUDE-Alerts website reduces the burden of pre/post market research by keeping you updated on the newest Medical Device Reports. We are confident the widespread adoption of this tool will make the medical device industry a safer place.
This website is kept up by the Innolitics developer team. If you use our tool, and would like to propose additional features to the site, please contact us with your suggestions!
We are currently accepting new software development projects! Please contact us if you need an expert team of engineers on your side.
Every great partnership starts with a conversation. Fill out the form below for a discovery call, and an Innolitics team member will contact you soon.