PhotoniCare

CEO and Co-Founder of PhotoniCare

PhotoniCare was building an optical-coherence tomography device to image the ear. Their team had deep hardware, optics, and firmware experience and one software engineer. This engineer was making slow progress, largely because they didn’t have experience with the medical device regulations and standards. It appeared the software may become the bottleneck on the project.
Innolitics was brought on to take over the software development and to provide necessary documentation for the first 510(k) submission.
Innolitics took over the software development and documentation. Software was no longer the bottleneck with the submission, and they achieved clearance (K191804) for the device.
We began by reviewing the existing software source code, including the LabVIEW prototype. We then worked with various stakeholders to understand the user needs and requirements for the production-grade software. We designed UI Mockups and an interactive prototype for the main screen and handheld screen of the device. These mockups were used to confirm all stakeholders approved of the design and that no key requirements were missed. We then built a software solution using C++, the Qt framework, and SWUpdate—in close conjunction with their firmware engineer.
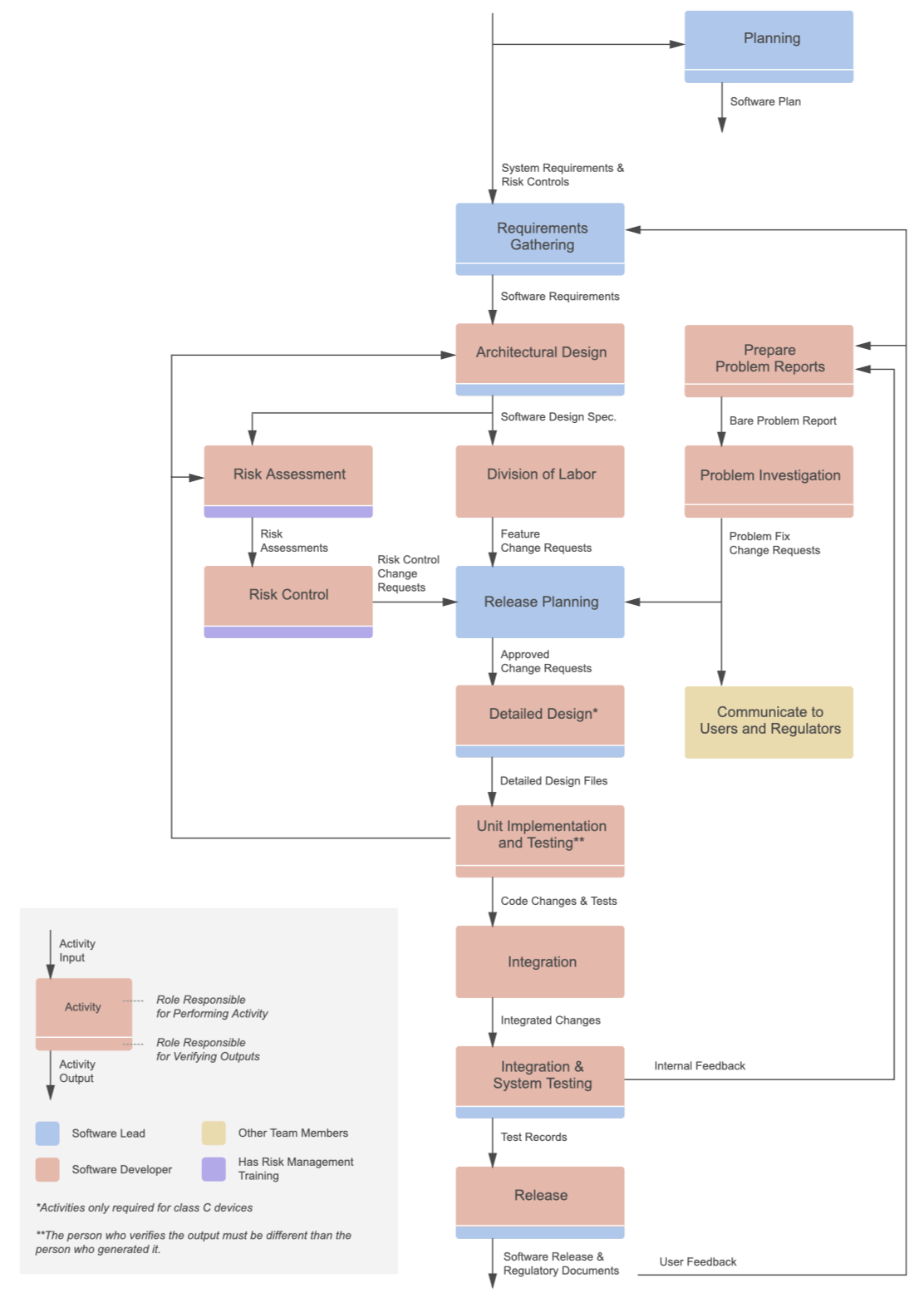
Our development was done following an IEC 62304-compliant process.
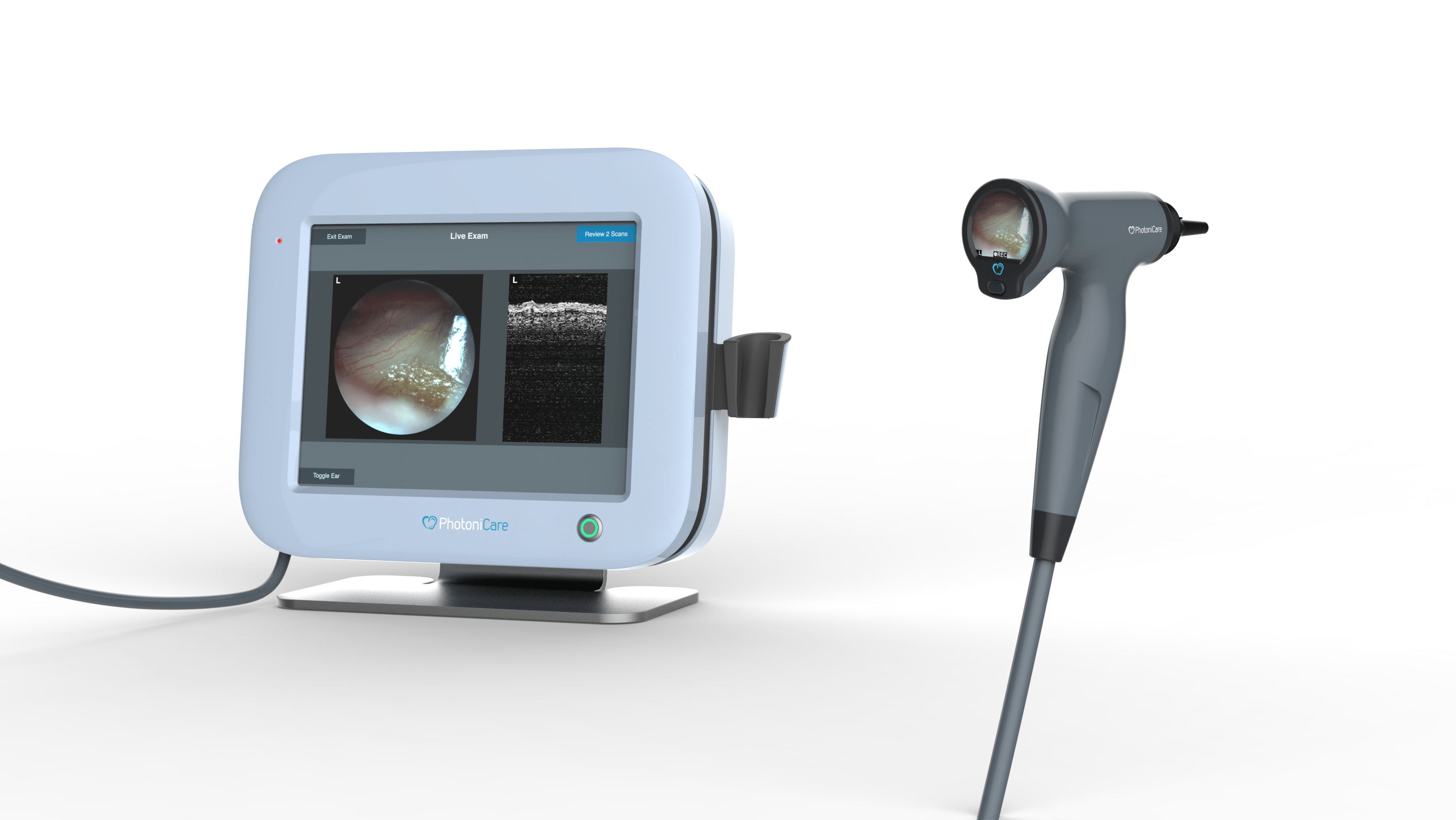
We continued to work on future versions of the software, including the development of an EMR integration, billing system, and more.
We also worked with PhotoniCare to win an STTR grant for an AI/ML component of their device. We’ve since worked with their team to analyze and build out this functionality.
Founded in 2013, PhotoniCare’s mission is to improve clinical outcomes and serve unmet patient & provider needs by translating novel light-based technologies into objective medical practice, starting with the OtoSight™ Middle Ear Scope. The FDA-cleared OtoSight™ Middle Ear Scope was developed for healthcare providers seeking a superior solution for pediatric middle ear issues. With remarkable 90+% reader accuracy in determining the presence or absence of fluid in the middle ear, the OtoSight™ Middle Ear Scope eliminates subjectivity and speculation by providing patients with a non-invasive and comprehensive assessment of middle ear fluid, even in the presence of significant earwax.
PhotoniCare has been recognized by numerous organizations, winning the 2020 SPIE PRISM award for medical technologies, the 2018 MedTech Innovator Execution Award, and the 2018 AdvaMed Accel Virginia Shimer Rybski Memorial Award. The company was also a member of the 2016 Dreamit Ventures accelerator program. PhotoniCare has received more than $6 million in support from the National Institutes of Health, most recently a Phase II SBIR award for work in artificial intelligence.

Dr. Shelton is a passionate and motivated business-focused engineer with a profound personal connection to the problem PhotoniCare is solving. He received a B.S. in Electrical Engineering from Oklahoma State University and a Ph.D. in Bioengineering from Texas A&M University, followed by three years of post-doctoral research at the University of Illinois. He has been building optical imaging systems for 20 years and managing engineering teams for 15 years. He leads PhotoniCare as its CEO and drives the company’s long-term vision and strategic plan. He has experience raising private financings, as well as negotiating and executing various research contracts, license agreements, distribution agreements, and other business development activities. He is passionate about his family, mountain biking, and music.
Every great partnership starts with a conversation. Fill out the form below for a discovery call, and an Innolitics team member will contact you soon.