It is not just a set of templates or an eQMS system, it is a full operating system to getting your device FDA cleared and maintaining your device in the post-market.
It is built on top of Notion, a world class knowledge management system used by big names like Figma, Pixar, Doordash, Nike, Amazon, GE, and Uber.
Notion is not only a joy to use for software engineers, it is also not a foreign language to non-engineers. Notion offers a familiar markdown interface with engineer centric features like code blocks and mermaid diagram rendering. At the same time, it has an intuitive interface that non-engineers can quickly learn.
We know because we have a team of 14 software engineers and 4 regulatory consultants that all use Notion simultaneously and harmoniously.
Did I mention we practice what we preach? We use Medtech OS on all of our engineering projects and even as our Innolitics QMS.
Medtech OS is our all-in-one workspace designed to streamline your medical device startup’s operations. Medtech OS goes beyond being a mere collection of templates; it integrates powerful project management tools to ensure seamless workflow and regulatory compliance. Drawing from our extensive expertise in both regulatory affairs and software development, we have crafted a robust and intuitive system that supports the different stages of your product lifecycle.
Starting from planning in Phase 1 all the way to validation and design transfer in Phase 4, Medtech OS’ Product Documents database contains all of the necessary templates for documenting a comprehensive Design History File (DHF) for your medical device. This ensures that every step of your development process is meticulously recorded, meeting regulatory requirements and supporting efficient project management.
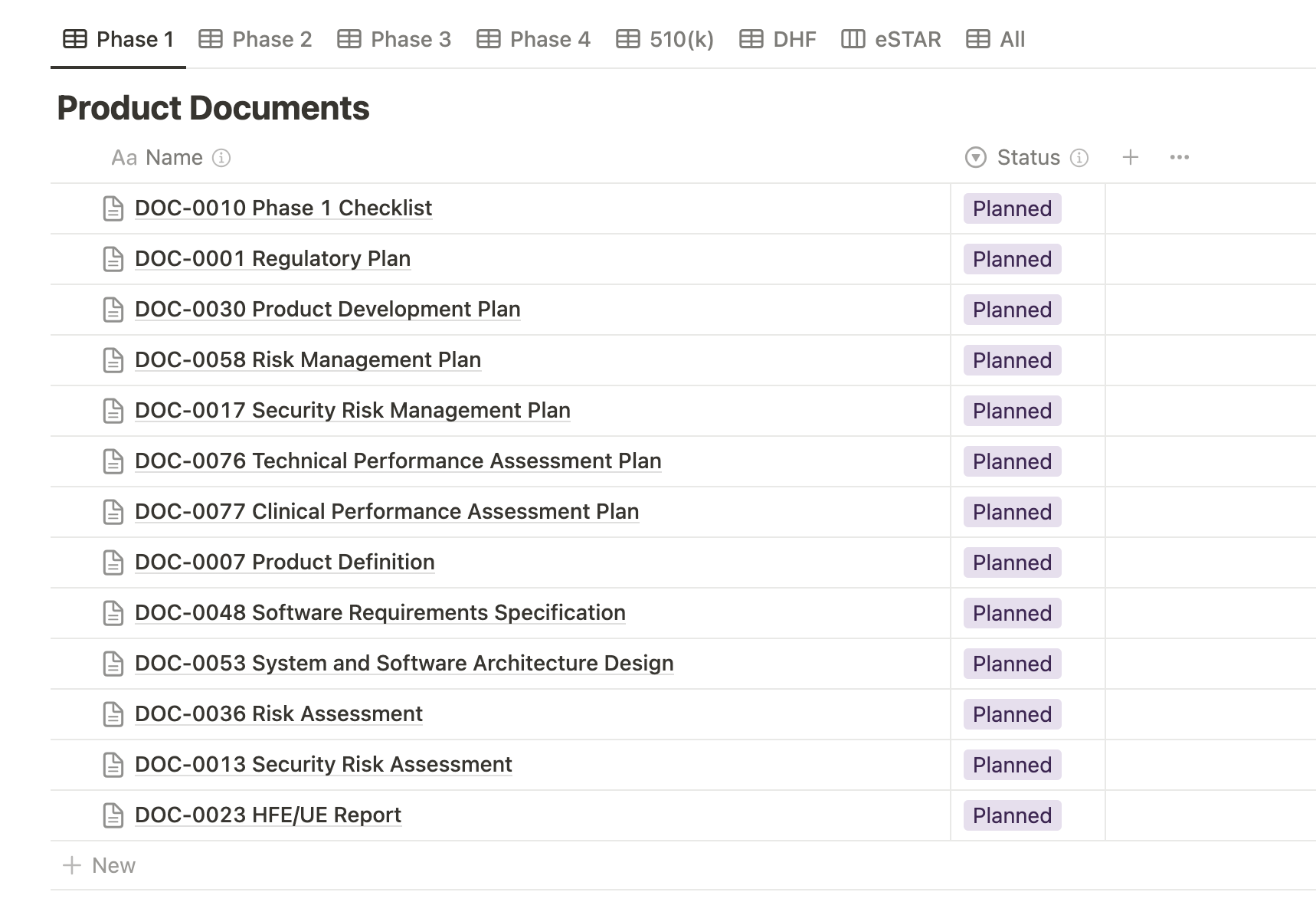
Our Phase Checklists do an excellent job at outlining all the documentation deliverables that are required to complete each phase of medical device development. These checklists provide a clear overview of the necessary documents produced at each stage and streamline collaboration by assigning specific tasks to team members. The checklist format ensures efficient tracking of completed items and outstanding tasks, enhancing project management and accountability throughout the development process.

Medtech OS provides the capability to filter views within the Product Documents database. The 510(k) view specifically highlights all the documents necessary for a 510(k) submission, ensuring a streamlined and organized process.
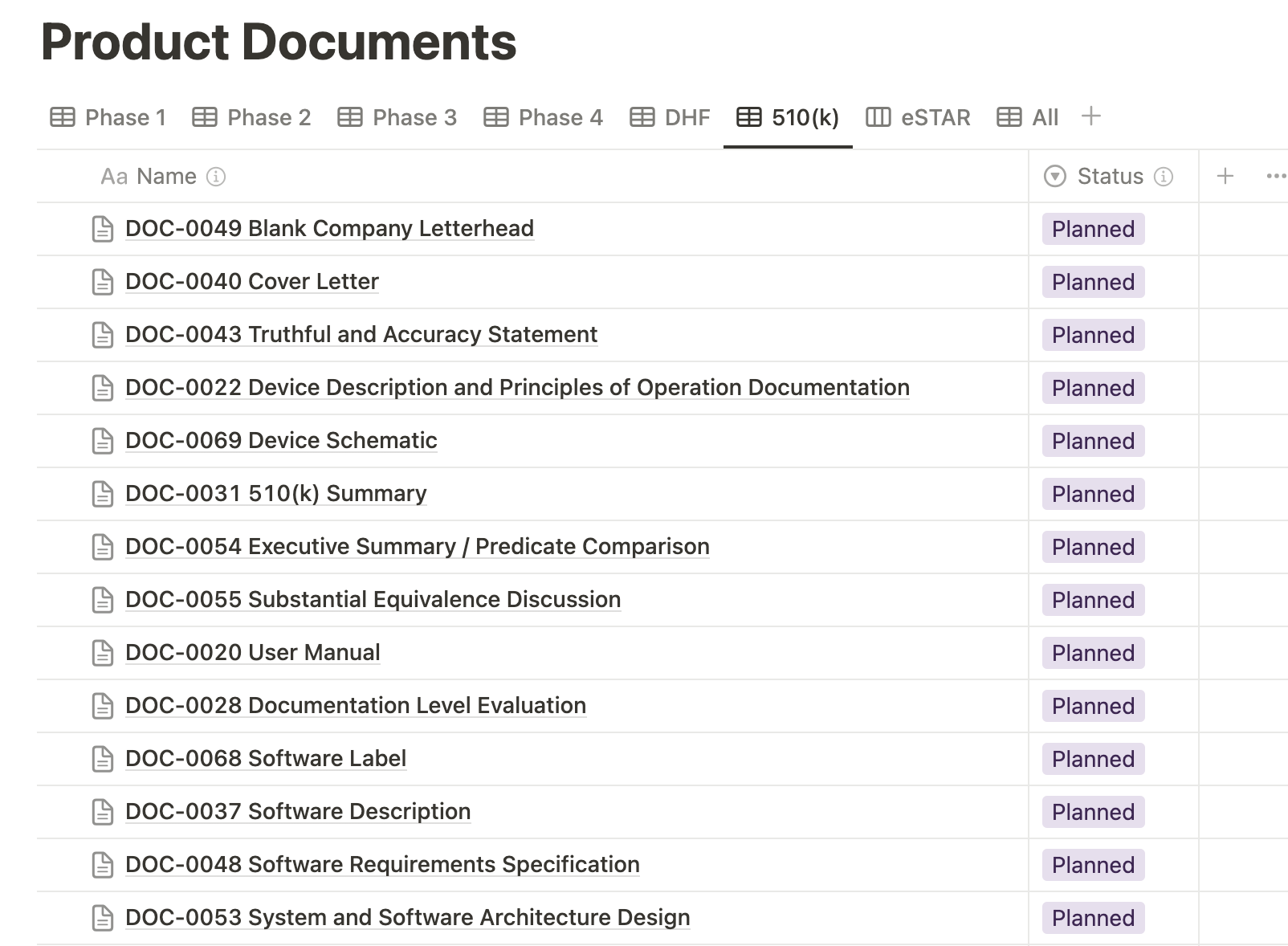
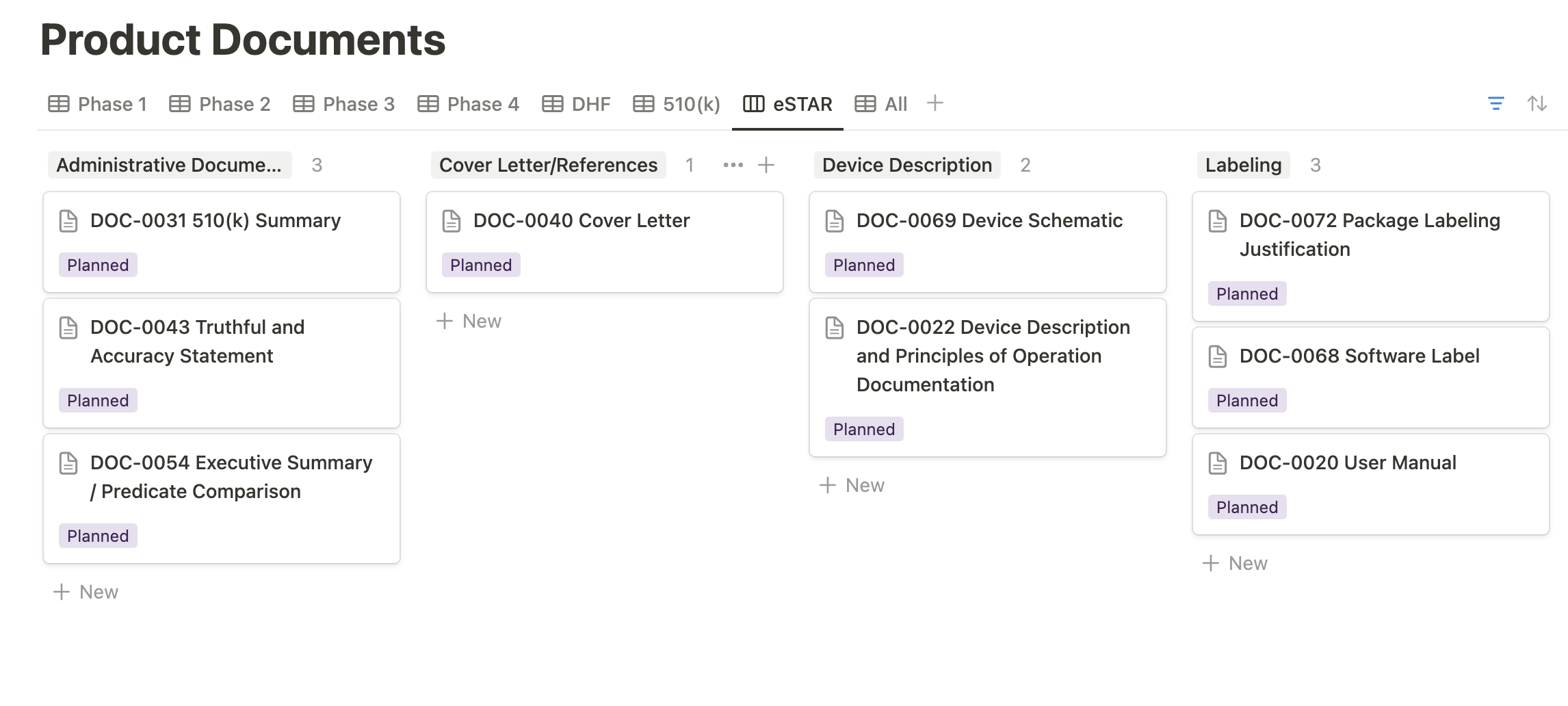
Similarly, the eSTAR view within our Product Documents database organizes the various 510(k) deliverables into their respective sections of the eSTAR. This feature enhances the visualization of your eSTAR submission status, providing a clear and organized overview for better management and tracking.
One of the standout features of Medtech OS is its ability to effortlessly maintain traceability across various databases and documents.
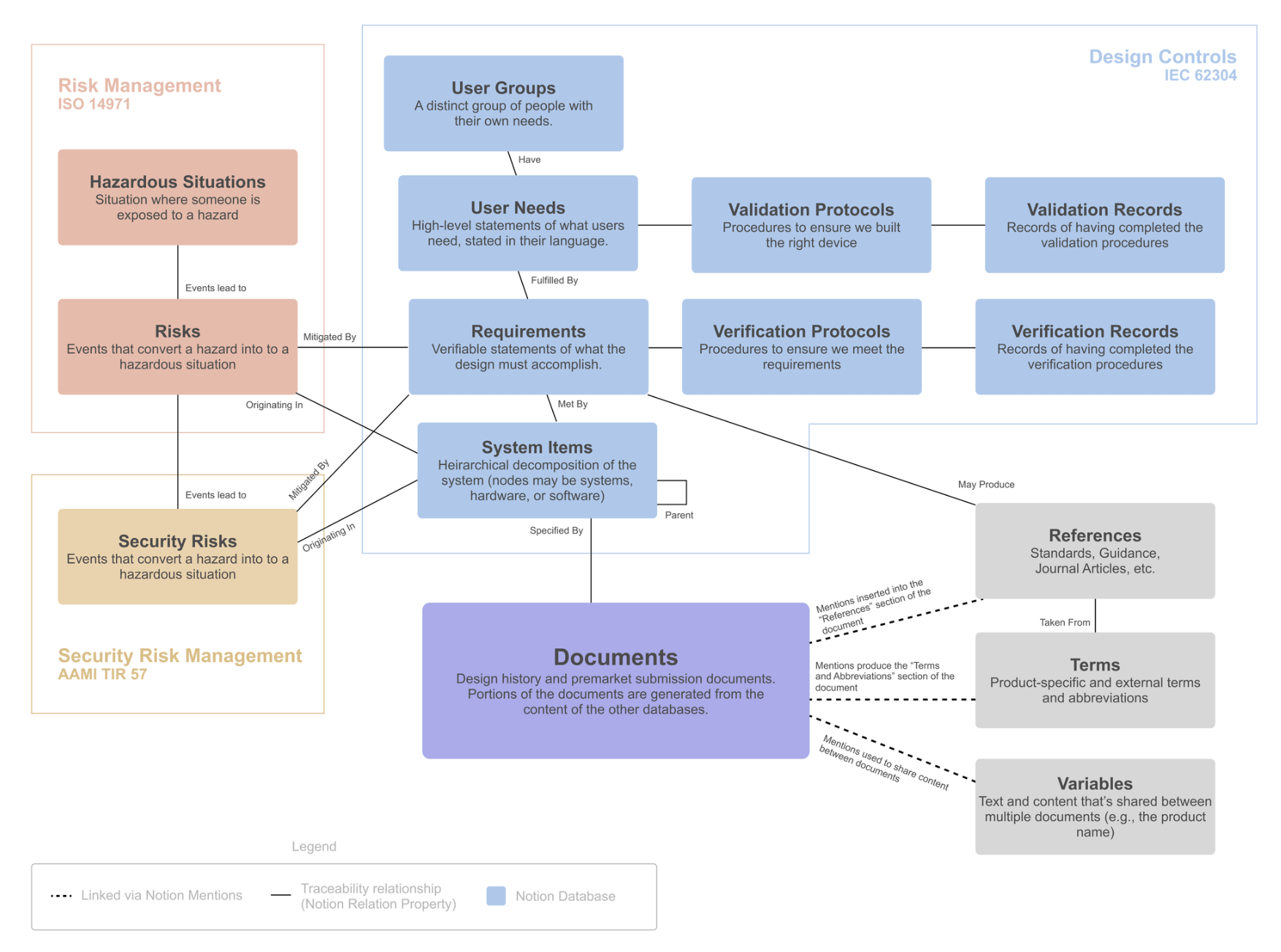
We have created multiple databases for each essential building block of a medical device, such as User Needs, Requirements, Risks, and Verification Protocols, among many others. Utilizing Notion’s relation property, we can effortlessly establish relationships between these elements, (No more navigating between spreadsheets to find the correct ID number!)
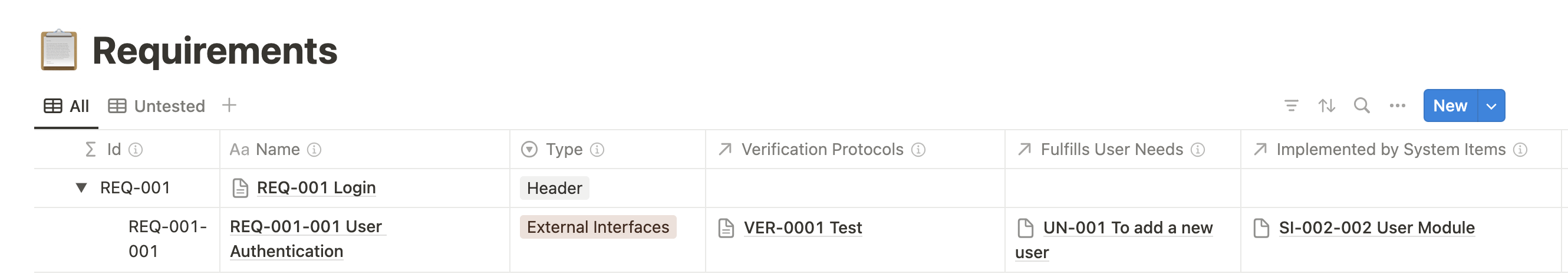
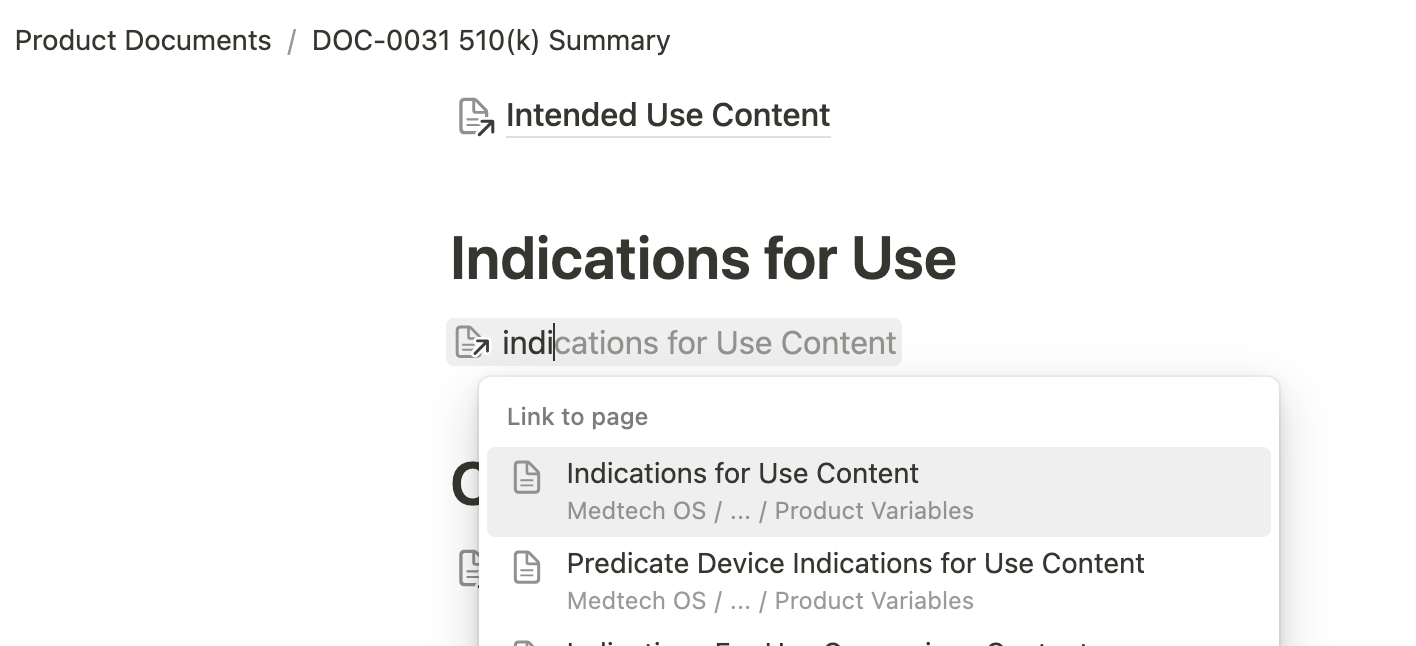
Another standout feature of Medtech OS is its ability to maintain consistency across numerous documents effortlessly. Have you ever had to update your Indications for Use across multiple documents or revise your entire submission just to change your device’s trade name? Our Product Variables database solves this problem by offering a centralized space for documenting specific content. These entries can be easily referenced across multiple documents using Notion’s @ mention feature. This not only simplifies content drafting but also ensures consistency, eliminating the risk of discrepancies across documents.
Notion offers a range of project management tools, including task management, timeline and calendar views, and to-do lists. Medtech OS seamlessly integrates these tools into the different Product Documents, simplifying and centralizing the management of your medical device project. This integration ensures that all aspects of your project are efficiently managed from a single platform.
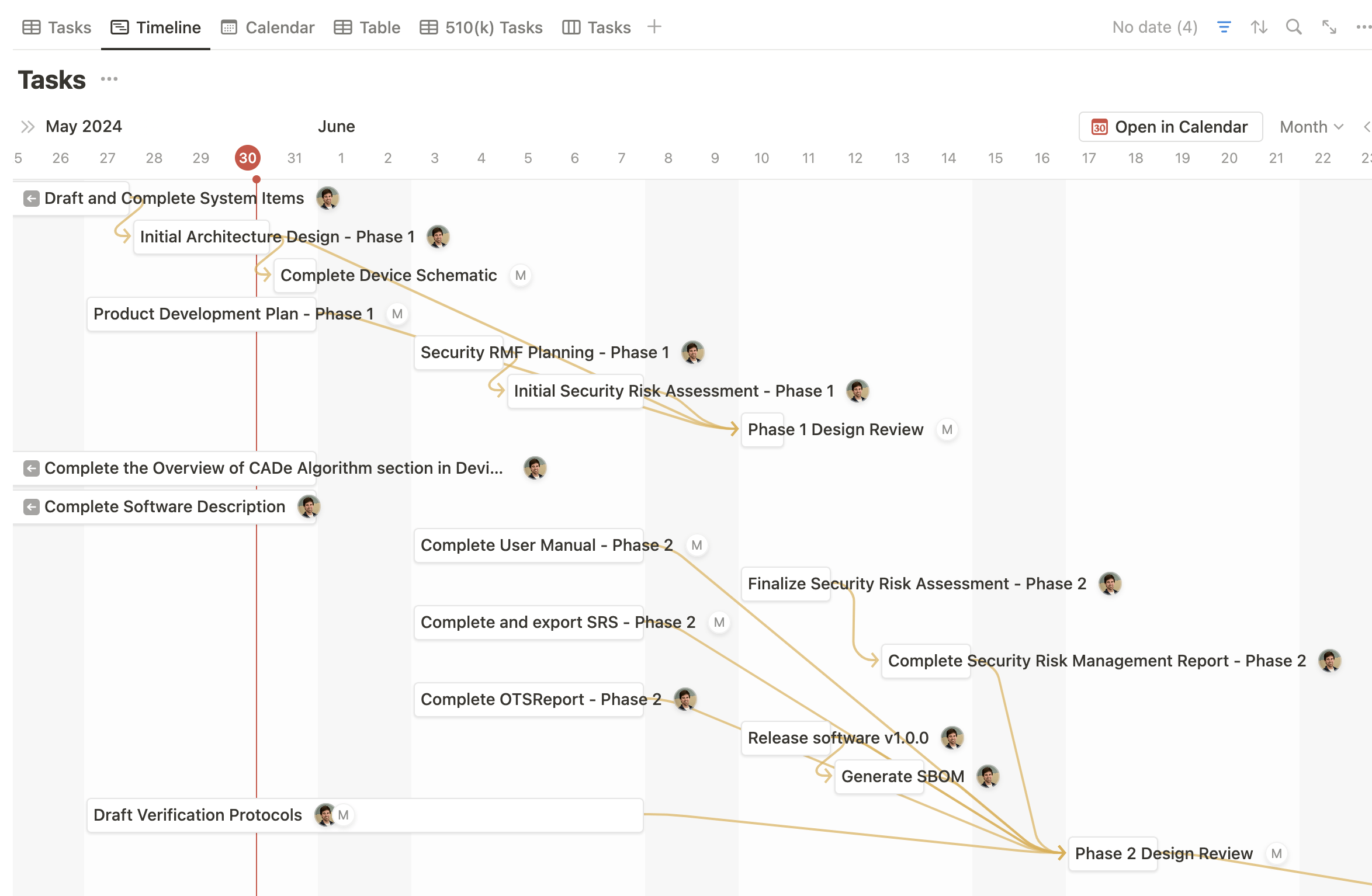
Life is not one-size-fits-all, and neither should your quality management system be. Medtech OS provides the perfect solution, enabling you to customize templates and processes to meet your unique requirements. For example, you can create filtered views of documents based on ownership or status. With Medtech OS, the possibilities are nearly endless, ensuring your quality management system is as adaptable as you need it to be.
| Medtech OS | Competitor Products | |
|---|---|---|
| Qualification Criteria | Varies | |
| Requirements Capture | Yes | Yes |
| ISO 14971 Risk Analysis | Yes | Yes |
| Substantial equivalence template | Yes | Yes |
| Device description template | Yes | Maybe |
| Verification and validation plan | Yes | Maybe |
| Test management system | Yes | Usually no. You require a third party test management system. |
| User manual template | Yes | No |
| IEC 62304 compliant software development plan template | Yes | Maybe |
| User needs | Yes | Yes |
| User groups | Yes | Yes |
| System items | Yes | Yes |
| V&V Capture | Yes | Yes |
| Unresolved Anomalies | Yes | Yes |
| Template updates in response to FDA changes. We keep up to date with the reg so you can focus on the dev. | Yes | No |
| CFR 820 part 11 compliant signatures | Yes | Yes |
| Document versioning | Yes | Yes |
| Document redline | Yes | Yes |
| Consulting | Yes | No. SaaS only |
| Everything you need to prepare and submit a 510k or De Novo | Yes | Yes but comes with a lot of additional baggage you don’t need until after FDA clearance |
| ISO 13485 Compliant QMS | Yes | Yes |
| CAPAs | Yes | Yes |
| Vendor Database | Yes | Yes |
| Learning management system | Yes | Maybe |
| Attribution | Optional | Varies |
| Case Study + Video testimonial | Optional | Varies |
| Innolitics submission | Optional | Varies |
| Traceability Management | Yes | Yes |
| DRY (do not repeat yourself) editing | Yes | No. You still need to mess with data duplication and playing intended use change whack-a-mole |
| UI that both engineers and non-engineers can understand | Yes | Either engineering loves it and regulatory hates it or vice versa |
| Cybersecurity templates | Yes | Varies. |
| Cybersecurity Consulting | Add-on | Usually separate vendors who may or may not work well together |
| All docs from a recently cleared 510k using Medtech OS | Add-on | No. This is unprecedented from only Innolitics. |
| Word Export | Yes | Yes |
| PDF Export | Yes | Yes |
| Automatic document signing workflow | Yes | Yes |
| Access control | Yes | Yes |
| Number of users | Unlimited | Limited by arbitrary caps |
| Epic / Sprint / Task / Issue management | Yes. Highly customizable. | Often in separate system |
| Software development consulting | Add-on | Usually separate vendors who may or may not work well together |
| AINN review | Add-on | Usually separate vendors who may or may not work well together |
| 510k Review | Add-on | Usually separate vendors who may or may not work well together |
| Generative AI Tools | Add-on | Varies but most implementations suck |
| Vendor Lock-in | No. Your data lives in Notion and Word document exports are available. | Yes. Usually difficult to export with APIs being locked. |
| APIs | Rich APIs available through Notion | Usually enterprise level feature |
| Specialized for SaMD | Yes | No. Often comes with confusing non-SaMD baggage like “shelf life.” |
| Step by step DHF preparation checklists | Yes | No. You are given the “what” and “why” but left on your own for the “how”. |
| Pricing model | Transparent | Suspenseful |
| User Limits | None | Arbitrary |
| Step by step 510k preparation checklist | Yes | No. Needs an external consultant. |
| Project management (Gantt charts, velocity, Scrum) | Yes | No. Needs separate tool. |
| Price | $10k initiation + $2.5k / quarter | Varies between $10k to $100k / year |
| Contact Us | Contact Us |
Every great partnership starts with a conversation. Fill out the form below for a discovery call, and an Innolitics team member will contact you soon.