Basic 🔗
What is ACNU Software? 🔗
ACNU stands for Additional Conditions for Nonprescription Use.
Under the ACNU regulatory rule, a “nonprescription drug product with an ACNU” is defined as an OTC drug product that could not be safely used by consumers based on labeling alone, but can be made safe and effective for OTC use if the sponsor implements an FDA-approved additional condition to ensure appropriate consumer self-selection and/or use.
As of October 2025, we’re not aware of any drugs on the market with an ACNU.
ACNU Software is software that enables an additional condition.

What are examples of ACNU software? 🔗
By ACNU software, we mean any software that is developed to implement the additional conditions for nonprescription use.
An ACNU can support appropriate self-selection, appropriate actual use, or both.
Appropriate self-selection means consumers decide the product is (or isn’t) right for them based on the labeling and any ACNU step(s). It tests the decision to use or not use. See FDA’s 2013 Guidance on Self-Selection Studies for Nonprescription Drug Products for more on this topic.
Appropriate actual use means consumers can actually use the product in real-world conditions—dose, frequency, duration, adherence, and they can self-diagnose/treat appropriately without a clinician.
Although FDA’s requirements are fluid, here are some hypothetical examples:
- A self-selection questionnaire
- An interactive educational module about appropriate use
- Software that accesses the patient’s medical records
- Software that identifies the patient and checks them against a registry
- Software that controls a drug distribution kiosk
- A mobile app that helps users manage their use of the drug
Would ACNU software be a medical device? 🔗
We believe in most cases, ACNU software would be a medical device.
First, we’d note that the word “device” confuses people because in everyday language it implies a physical thing. In regulatory language, the term “device” includes software. Software-only devices are referred to as “Software as a Medical Device”, or SaMD.
Software that is “intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease” is considered a medical device.
Software that supports an ACNU would typically be intended for the use in the treatments or prevention of a disease, namely through helping ensure the safe use of the drug.
The regulatory rule itself doesn’t discuss this topic, however, a question and response on the rule provides a bit of insight int
Does ACNU software need to comply with FDA’s cybersecurity guidance? 🔗
Yes. We would expect ACNU software, whether it is a standalone device or part of a combination product, would need to follow FDA’s cybersecurity requirements.
You can read much more about this topic in our in-depth article titled Medical Device Cybersecurity: Best Practices, FAQs, and Examples.
Why did FDA developer the ACNU regulation? 🔗
FDA has been exploring ways to enable more prescription drugs to transition to OTC for over a decade. This regulatory rule is the culmination of these efforts.
What regulations and guidance apply to ACNU software? 🔗
See our article Medical Device Software: Best Practices, FAQs, and Examples for an in-depth discussion of this topic.
In brief, we’d expect the need for the software to undergo a De Novo or 510(k) submission, and to be comply with the following guidance:
- 2019 FDA Guidance - Policy for Device Software Functions and Mobile Medical Applications
- 2025 FDA Guidance - Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions
- 2023 FDA Guidance - Content of Premarket Submissions for Device Software Functions
- 2023 FDA Guidance - Off-The-Shelf Software Use in Medical Devices
- 2020 FDA Guidance - Multiple Function Device Products: Policy and Considerations
- 2016 FDA Guidance - Postmarket Management of Cybersecurity in Medical Devices
- 2016 FDA Guidance - Applying Human Factors and Usability Engineering to Medical Devices
- 2014 FDA Guidance - Design Considerations for Devices Intended for Home Use
Should we build or buy our ACNU software? 🔗
This decision depends on several factors including your timeline, budget, internal capabilities, IP control needs, and strategic goals. Building custom ACNU software gives you complete control over features, IP, user experience, and regulatory strategy.
Buying an existing solution can accelerate time-to-market, though you may have less flexibility in customization and could face vendor dependencies.
For example, if FDA requires that your ACNU software include certain safety controls, you would be at the mercy of the ACNU software vendor to implement these controls.
Most software-development companies do not have experience with medical device regulations. Therefore, if you do decide to develop your own ACNU software, be sure your developer can support your regulatory needs.
Does Innolitics support ACNU software development? 🔗
Yes. Our team of software engineers, cybersecurity experts, and FDA regulatory experts can fully support pharmaceutical companies developing ACNU software.
We can work with your regulatory team to identify the appropriate strategy, support FDA interactions, develop the software, and validate the software. Our process complies with all of the guidance listed in the previous question.
Depending on the range of your needs, we can develop custom ACNU software that fully complies with FDA’s regulations and guidance in 3-6 months. Our speed is made possible by our integrated team and experience developing over 50 SaMD applications.
Where can I find more information? 🔗
- The finalized regulatory rule from December 2024.
- An ONPD webinar (and PDF slides) from February 2023
- An interesting Skadden article about ACNU commercialization from January 2025
Examples 🔗
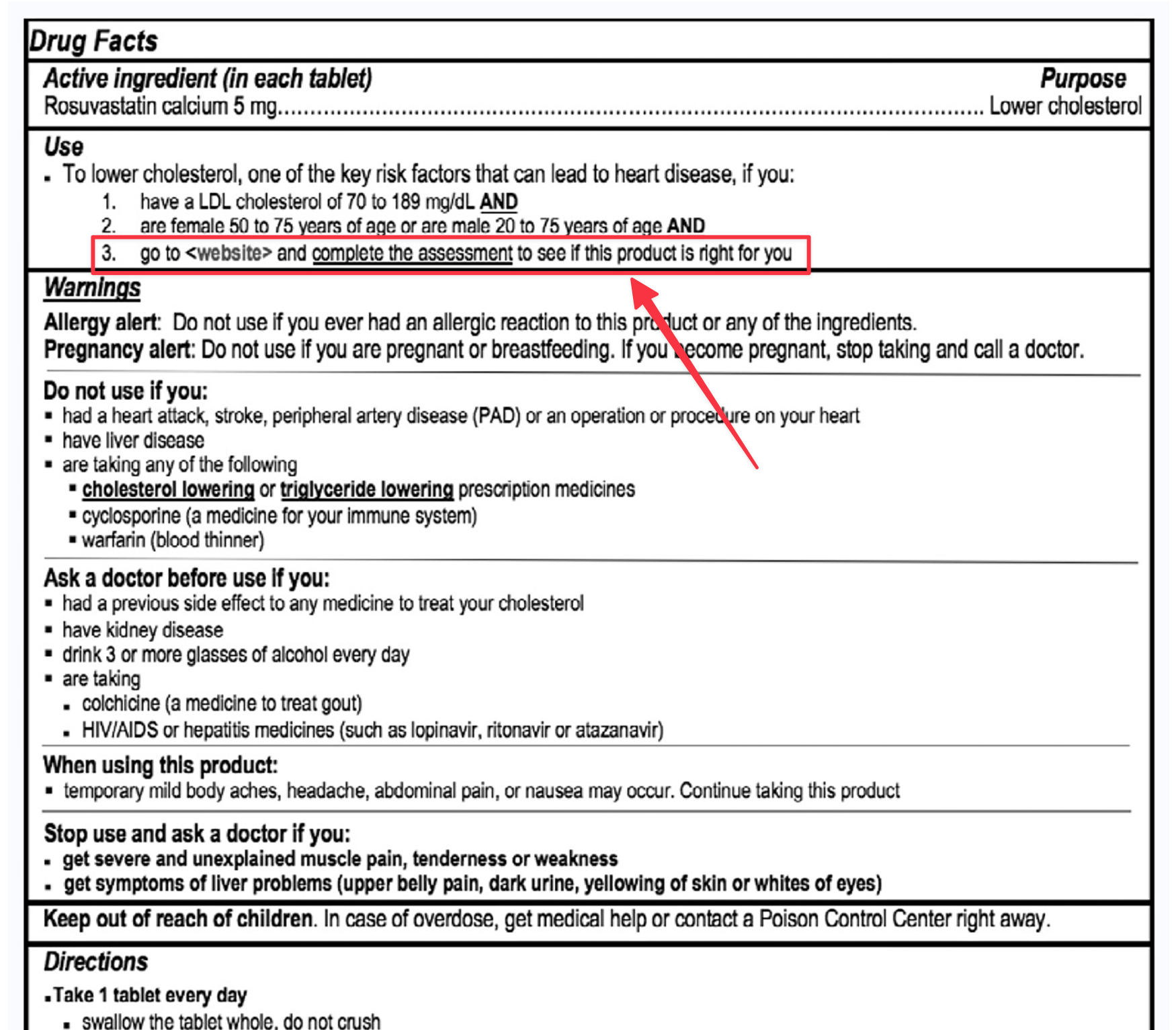
A Technology-Assisted Web Application for Consumer Access to a Nonprescription Statin Medication 🔗
Although statins reduce cardiovascular morbidity and mortality, only about one-half of eligible patients receive treatment. Safe and appropriate consumer access to statins could have a significant positive public health impact.
Concentrics Research, AstraZeneca, Idea Evolver, and C5Research conducted a self-selection and actual use study of a statin drug and web-app combination product.
The full study is quite interesting to read and can be found here. Here are a few screenshots of a Software as a Medical Device web-app that supports an additional condition:
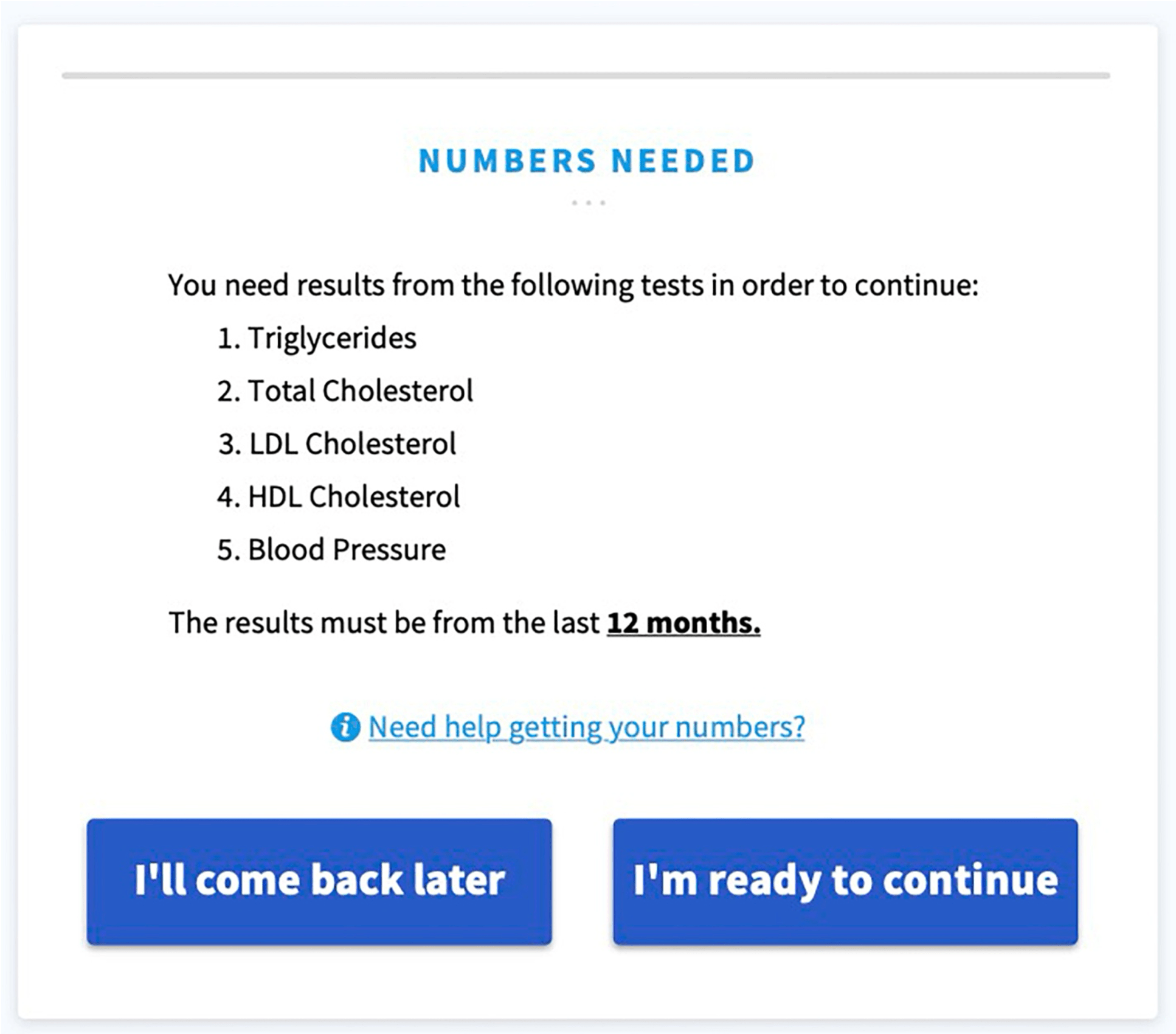
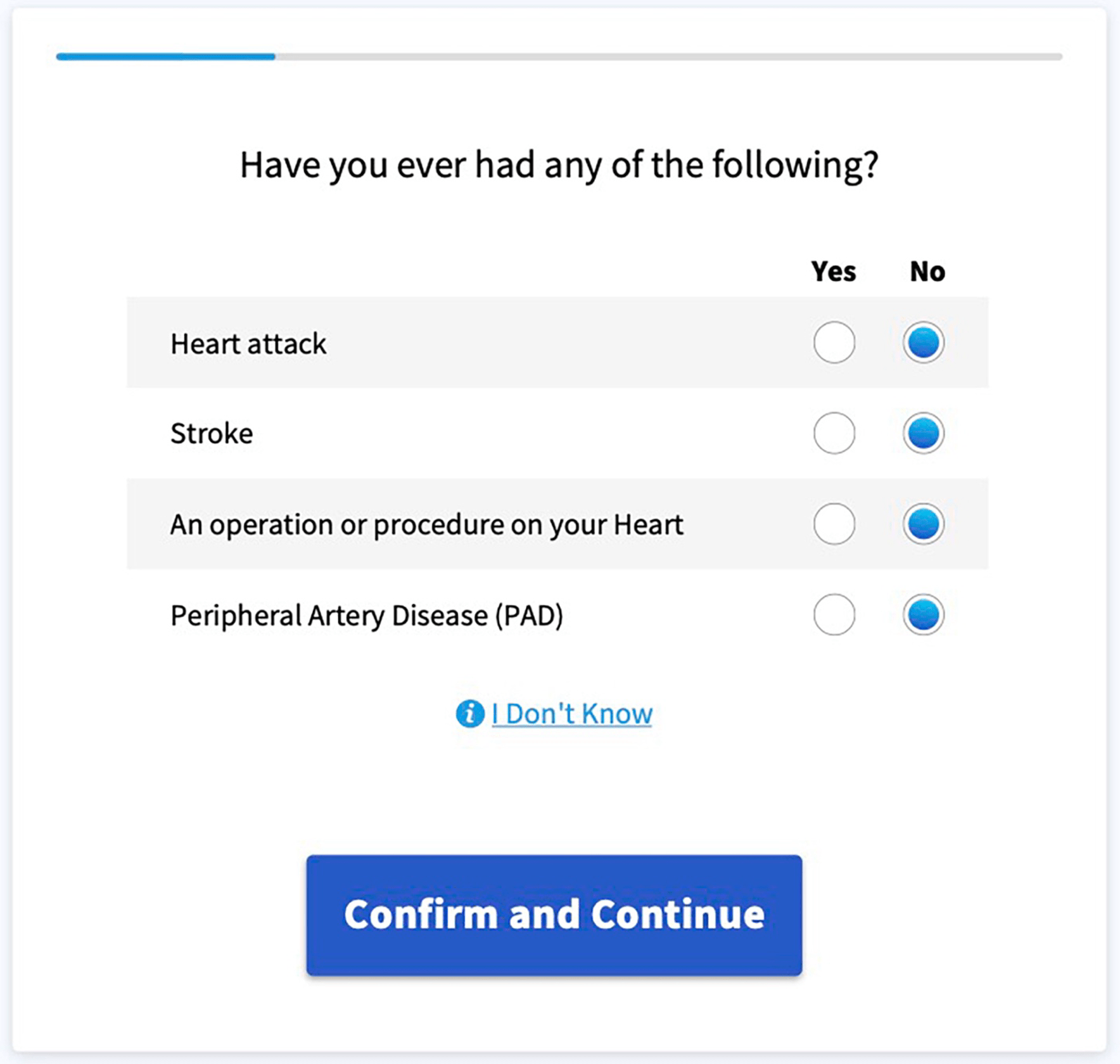
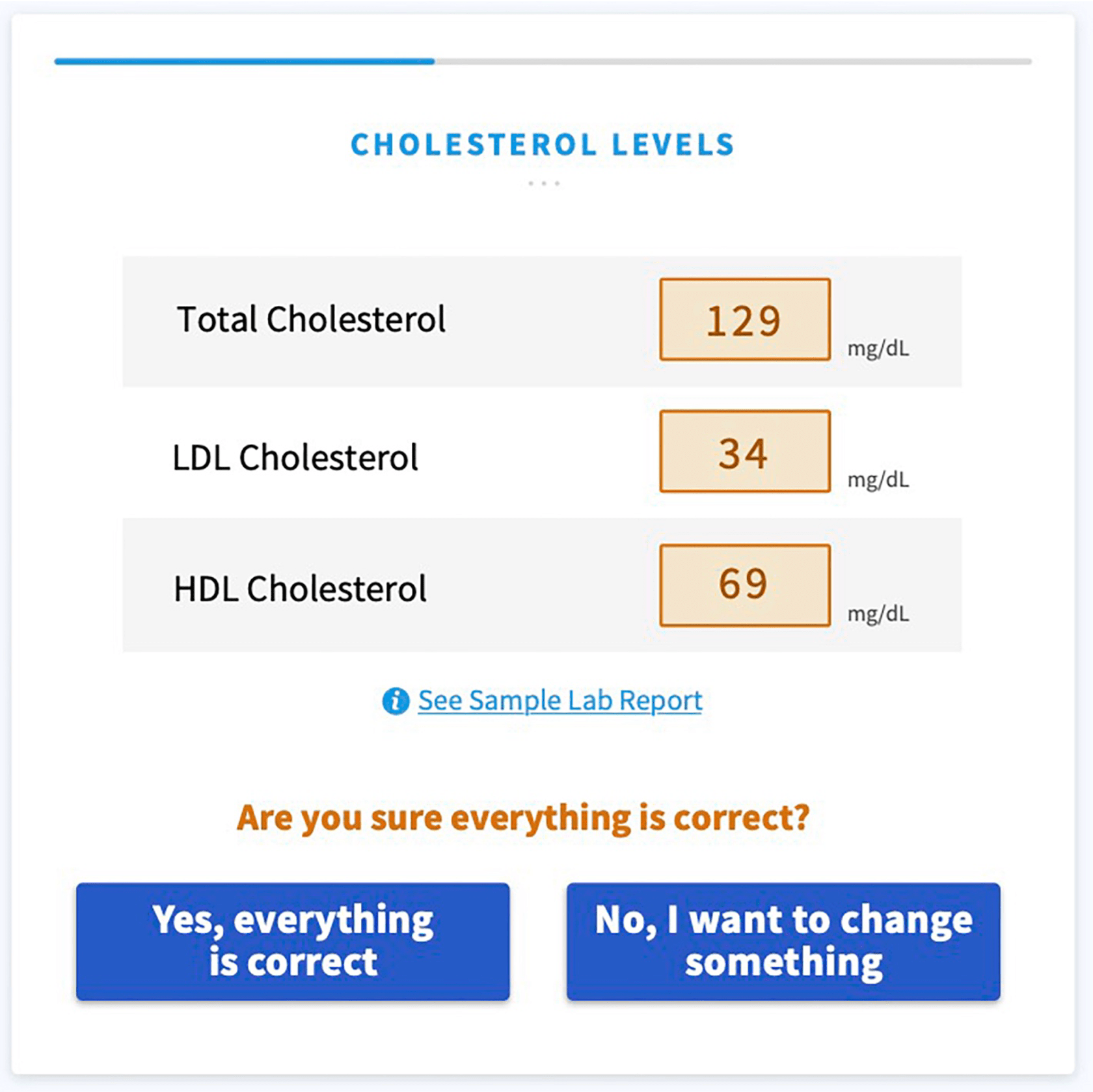
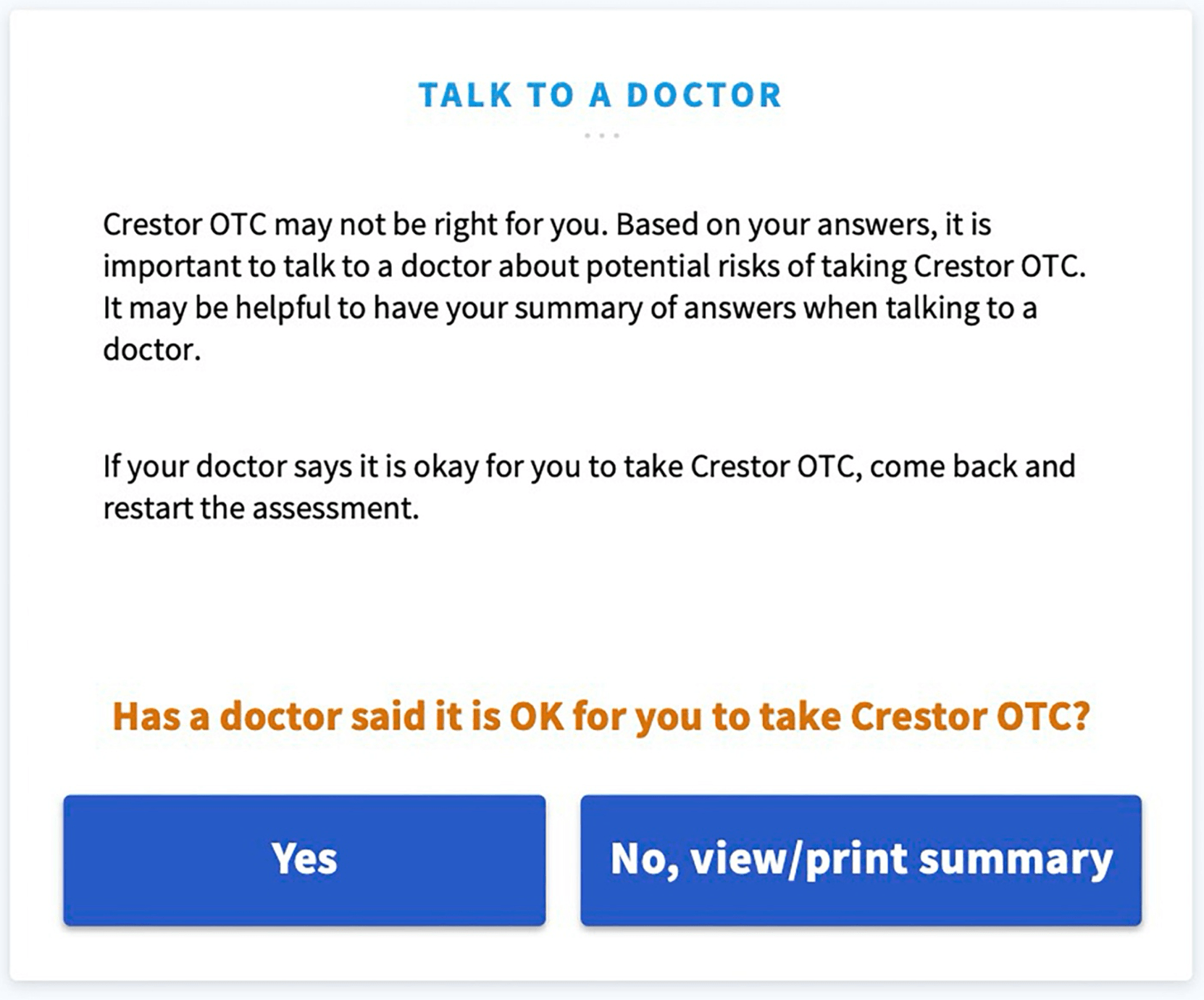
Revision History 🔗
| Date | Author | Changes |
|---|---|---|
| 2025-10-28 | J. David Giese | Initial Version |

