Introduction 🔗
The FDA has become increasingly focused on medical device cybersecurity, as can been seen by the frequency and length of their guidance (2014, 2016, 2018, 2022, 2023, and 2024). This has only accelerated when, in 2023, congress gave FDA additional authority to regulate the cybersecurity of medical devices.
For groups who are less experienced with cybersecurity, reading the FDA guidance and the various standards can be overwhelming. Fortunately, when you boil down these abstract documents into the concrete actions you need to take, it’s actually much simpler. In this article, we collect a variety of best practices, questions, and examples related to cybersecurity. Our goal is to be as concrete and specific as we can.
We’ve also seen a large up-tick in the FDA’s requests for additional information in medical-device submissions.
If you like this article, you may also be interested in our other “FAQ, Best Practices, and Examples” style articles:
- Medical-Device Software Regulations: Best Practices, FAQs, and Examples
- Design Inputs: Best Practices, FAQs, and Examples
- SBOMs: Best Practices, FAQs, and Examples
If you have medical-device cybersecurity questions that aren’t answered here, feel free to reach out to us in the website chat and we’ll get back to you.
We are a software development and regulatory consulting firm that specializes in SaMD. We help manufacturers with cybersecurity, regulatory strategy and submissions, and provide end-to-end SaMD development.
About the Author 🔗

Hi, I’m David Giese, a Partner at Innolitics. I’ve wrote this article based on my real-world experiences bringing 35+ diagnostic medical devices onto the US market. I take pride in the in-depth articles I write, and appreciate feedback and questions. I’d love to connect on LinkedIn, where I post pragmatic tips about medical-device cybersecurity for my >5k followers.
FAQs 🔗
Please note that threat modeling is used in many industries. This article discusses threat modeling medical devices and focuses on the US regulations.
Basics 🔗
What is cybersecurity? 🔗
With regards to medical devices, the FDA defines cybersecurity as the process of preventing
- unauthorized access,
- modification,
- misuse or denial of use, or
- the unauthorized use of information that is stored, accessed, or transferred from a medical device to an external recipient.
Do all medical devices need to comply with the FDA’s cybersecurity guidance? 🔗
Per FDA’s eSTAR PDF template, you need to handle cybersecurity if your device
- Is hosted in the cloud
- Has a network connection (active or not)
- Has wireless communication of any form
- USB, serial ports, or removable media
- Support software upgrades (including patches).
If you meet any of these conditions, FDA will require you to provide cybersecurity documentation in your 510(k) submissions and have a plan for monitoring, assessing, fixing, and reporting vulnerabilities after your device is on the market.
Technically, here’s the regulatory definition of a “cyber device”:
In our experience interacting with the FDA, they interpret “has the ability to connect to the internet” broadly and if you meet any of the conditions in the eSTAR template, you’ll have a hard time arguing you’re not a cyber device. For example, devices that only have a USB port are still considered cyber devices. So are docker images which do not connect to the internet but are expected to be run in an environment where they can connect. For example, here’s a response from the FDA when we had tried to argue a device with just a USB port is not a cyber device:
What cybersecurity documents are required for a 510(k), De Novo, or PMA? 🔗
The same set of cybersecurity documents are required for all three types of FDA submissions, however, the level of detail scales with the security risk level of your device. Here is the full list of documents from the latest v5.5 eSTAR:
| Document | Summary |
|---|---|
| Security Risk Management Report | Final summary of all security activities providing submission-ready overview, including a description of your separate, parallel, and interconnected security risk management process. |
| Threat Model | E.g., a STRIDE analysis of threat actors, assets, and attack vectors, showing potential vulnerabilities and safety impacts. |
| Security Risk Assessment | Risk evaluation showing traceability between vulnerabilities, controls, and residual risks. |
| Software Bill of Materials (SBOM) | List of all software components and third-party libraries used in the device. Seefor details. |
| SBOM Support Report | A document showing the support duration and end-of-life plans for each SBOM component. |
| Vulnerability Assessment | Review of vulnerabilities found in SBOM scan. |
| Assessment of Unresolved Security Anomalies | Open security issues with impact analysis and mitigation plans. |
| Cybersecurity Measures and Metrics | Indicators tracking security control effectiveness (e.g., vulnerability count, patch response time). For new devices, outlines planned metrics only. |
| Cybersecurity Controls | Proposed and final security controls addressing FDA's 8 risk control categories to mitigate identified risks. |
| Security Architecture Views | Visual diagrams of system components, data flows, connections, and trust boundaries. Includes Global System View, Multi-Patient Harm View (if applicable), Use Case Views, and Updateability View. |
| Cybersecurity Testing Report | Summary of all security testing activities and results. |
| Penetration Testing Report | Third-party pen-test findings and recommendations with FDA-aligned analysis. |
| Cybersecurity Labeling | User documentation covering security responsibilities, diagrams, updates, and anomaly reporting. |
| Cybersecurity Management Plan | Plan for managing cybersecurity risks throughout product lifecycle, including development, vulnerability handling, and monitoring. |
What cybersecurity activities must be done when creating a medical device? 🔗
The guidance suggests incorporating security into the full development life-cycle. The FDA guidance is written in a somewhat abstract way. Here, I’ll list the key activities as they fall within a typical software development lifecycle process:
- Planning
- Identify the personnel responsible for security activities
- Define how the exploitability, severity, and acceptability of security risks will be assessed
- Identify the methods that will be used to identifying security risks, including at least threat modeling
- Identify the threat-modeling methodology that will be used
- Identify any secure coding standards or static code analysis tools that will be used during development
- Plan for how the development environment will be kept secure (e.g., requiring two-factor authentication in GitHub)
- Plan for how software updates will be delivered in a timely fashion to the device
- Architecture
- Document key security views (these will be used in threat modeling)
- Security risk assessment - given the initial architecture, analyze likely security risks
- Development
- Implement the security controls that are identified through the security risk assessment
- Labeling
- Generate an SBOM to be shared with customers (and used to monitor for vulnerabilities)
- Cybersecurity documentation in labeling
- Design Verification
- Penetration Testing
- Vulnerability Testing
- Verification of all security controls
- Design Validation
- If applicable, validate that customer IT Staff have sufficient information in labeling to understand their security responsibilities
- Maintenance
- Reporting security vulnerabilities that are identified
- Monitoring your SBOM for vulnerabilities
- On-going vulnerability testing
- On-going penetration testing
- Security updates
Security Risk Management 🔗
What is security risk management? 🔗
Security risk management is similar to safety risk management. Both attempt to identify “bad things” that could happen, assess how likely or severe they would be if they did happen, and then mitigate them from happening. They differ in a few key ways. First, the types of “bad things” are different. Thus, the severity level definitions and acceptance criteria are different. Second, in the case of cybersecurity, there may be a malicious actor attempting to make the bad things happen for some motive. Thus, probabilistic analysis of failure is less relevant.
How is security risk management related to safety risk management? 🔗
Safety risk management focuses on harm to patients and device users. Security risk management considers harm to assets due to cybersecurity breaches more generally, including business reputations, PHI, and other assets not accounted for in safety risk management. (As an aside, risk management is useful to model anytime you’re trying to anticipate harm more broadly, including harm to business profitability, regulatory submission risk, and so on.)
Security risks are sometimes, but not always, related to patient safety risks.
Safety risk management is clearly defined in the widely-adopted ISO-14971 standard.
Security risk management for medical devices is described in the AAMI TIR57 “Principles for medical device security—Risk Management” Technical Information Report, which the FDA has recognized as a consensus standard.
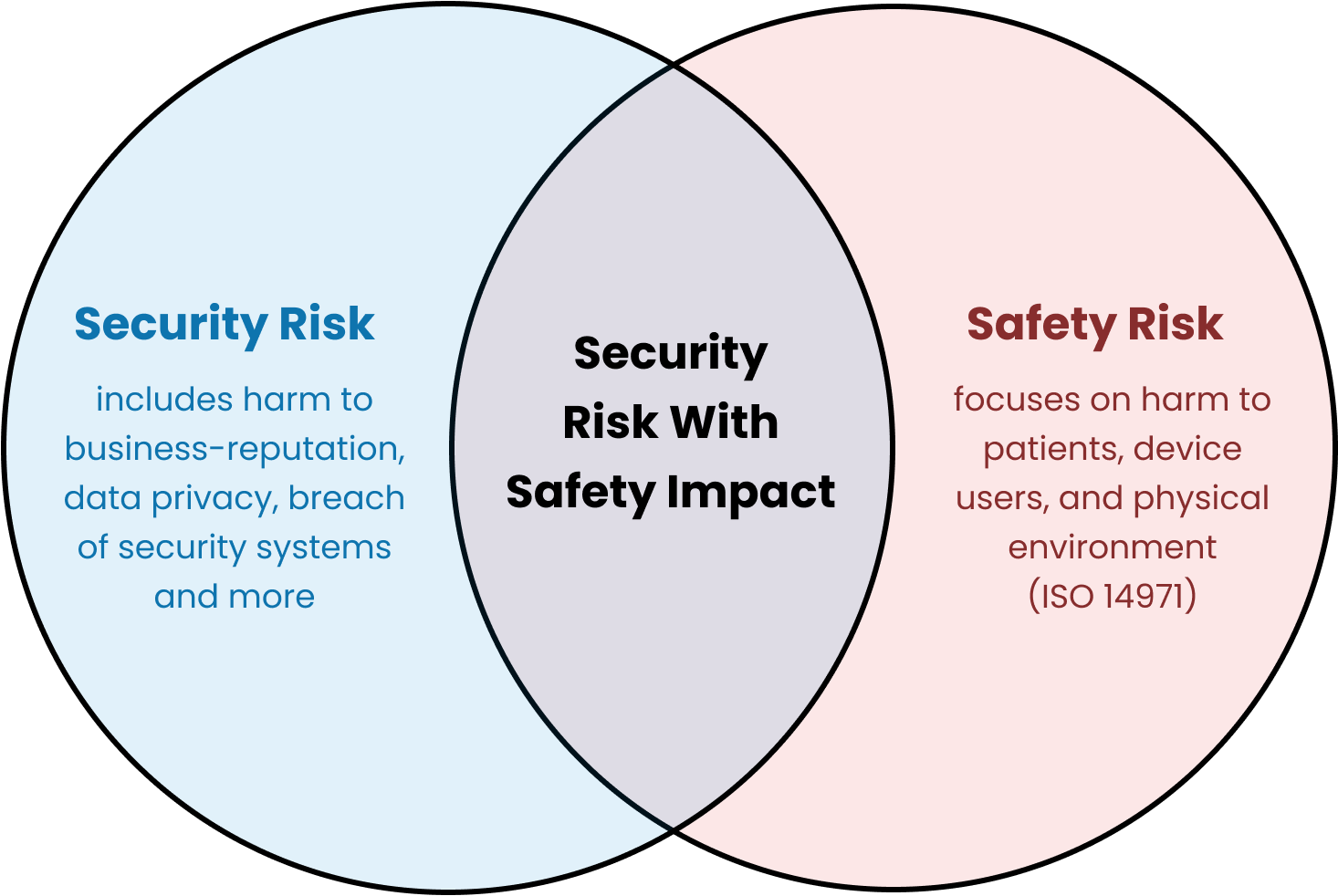
There is a very useful diagram, which I’ve copied from TIR57, that shows the relationship between the processes:
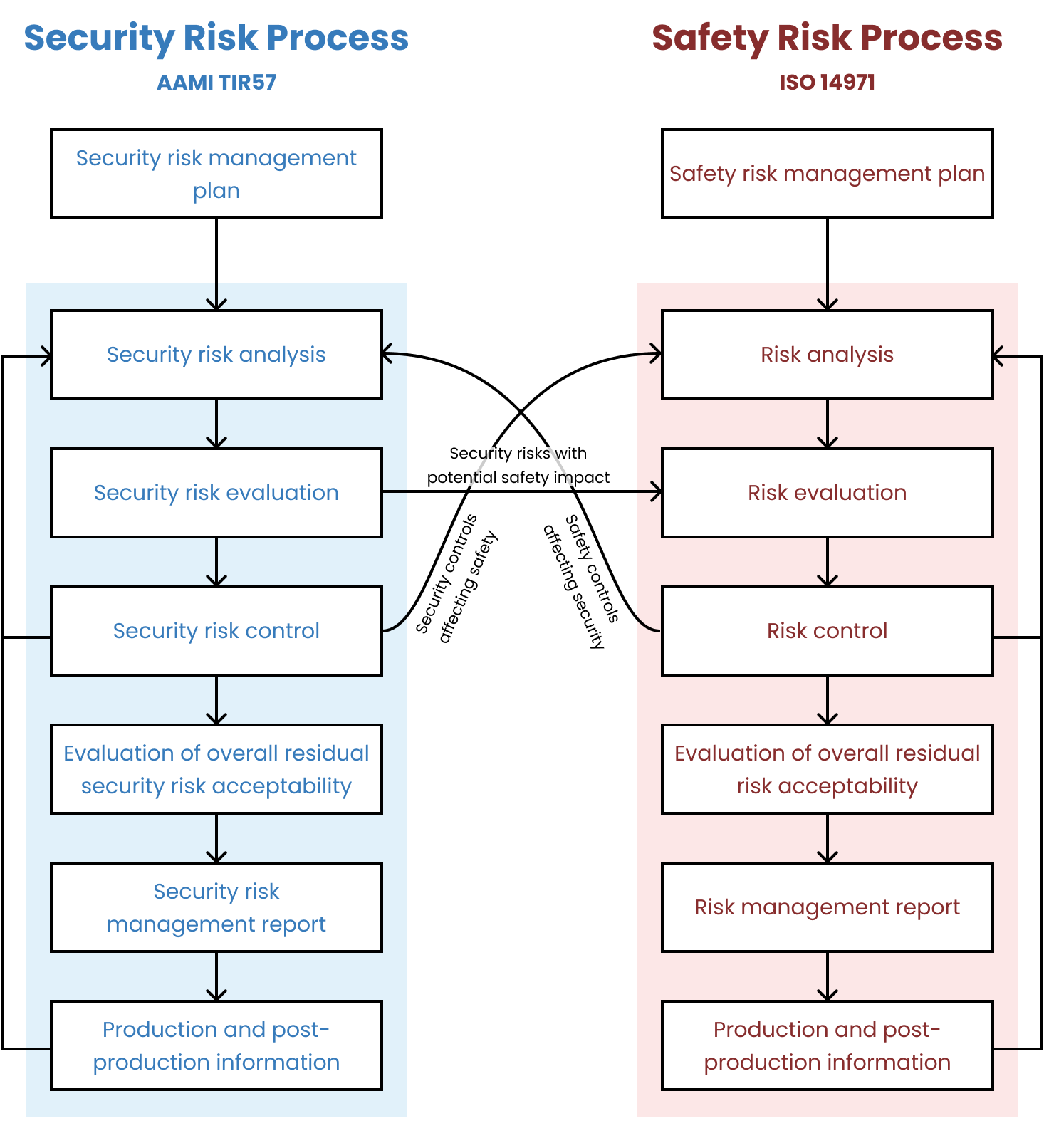
Is it okay to incorporate security risk management into safety risk management? 🔗
No. Previously, FDA had been more open to combining the two processes and we’d suggested (for simple devices) that our clients keep them combined. FDA has been clear in their 2023 guidance and in their newest eSTAR templates that the two processes need to be separate:
How should security risks be assessed? 🔗
This is a complicated question. We’ve seen some companies use the Common Vulnerability Scoring System (CVSS). For most devices, we don’t suggest that CVSS be used out of the box as it is too complicated (e.g., see the CVSS Version 4.0 Calculator).
We assess security risks following a security risk-acceptability table with two axes along exploitability (not likelihood!) and severity. The tables below present the levels along each of these two access, which combine to present the overall acceptability. This is much like its done for ISO 14971.
The specifics of these tables should typically be modified for your particular device and use environment. Here is an example approach that we have used on multiple FDA submissions:
Security Exploitability Levels
The exploitability of a security risk occurring shall be classified according to the proximity of access and the ease of exploitation required. The overall exploitability level shall be the mean score of these factors, rounded up.
| Label | Proximity of Access | Ease of Exploitation |
|---|---|---|
| Negligible (1) | Physical access requiring long-term or repeated hands-on contact. Typically Local (L) CVSS Access Vector. | Highly specialized conditions and tools required or multiple independent authentication factors (e.g., hardware key + password). Typically High (H) CVSS Access Complexity. |
| Minor (2) | Temporary physical access to device. Typically Local (L) CVSS Access Vector. | Some specialized equipment or conditions required or multi-factor authentication (e.g., password + PIN). Typically High (H) or Medium (M) CVSS Access Complexity. |
| Moderate (3) | Private network (e.g., hospital LAN). Attacker must be on same subnet or have limited logical access. Typically Adjacent (A) CVSS Access Vector. | Some specialized knowledge or environment needed. Single-factor authentication (e.g., password or badge). Typically Medium (M) CVSS Access Complexity. |
| High (4) | Local or short-range network or RF (e.g., Bluetooth). Typically Adjacent (A) CVSS Access Vector. | Few specialized tools, widely known vulnerabilities, or simple single-factor or weak credential (short PIN). Typically Low (L) CVSS Access Complexity. |
| Very High (5) | Remote access required (e.g., internet). Typically Network (N) CVSS Access Vector. | No authentication or protections and little to no specialized conditions required. Typically Low (L) CVSS Access Complexity. |
Security Risk Severity (Impact) Levels
The severity (i.e., impact) of a security risk occurring shall be classified according to these qualitative levels:
| Label | Impact Description |
|---|---|
| Negligible (1) | No negative effects to device operations, business operations, data, or other organizations/environment |
| Minor (2) | Temporary inconvenience to device operation, minor customer complaints, loss of non-confidential data, or device revealing unnecessary information about itself |
| Moderate (3) | Single device availability loss or tampering, damage to business reputation, loss of non-essential data integrity or small amount of confidential data, or device becomes an information gathering vector |
| Serious (4) | Multiple device availability loss, serious legal issues, loss of essential clinical performance, large confidential data breach, or the device becomes an attack vector against other systems |
Security Risk Acceptability Levels
| Label | Description |
|---|---|
| Acceptable | The security risk level is acceptable. |
| Attempt to Mitigate | The risk level is acceptable, but risk control measures that are feasible to implement within time and cost constraints should be implemented. |
| Unacceptable | The risk is unacceptable and must be mitigated. If it can’t be mitigated further, then a risk/benefit-analysis must be performed. |
Security Risk Acceptability Matrix
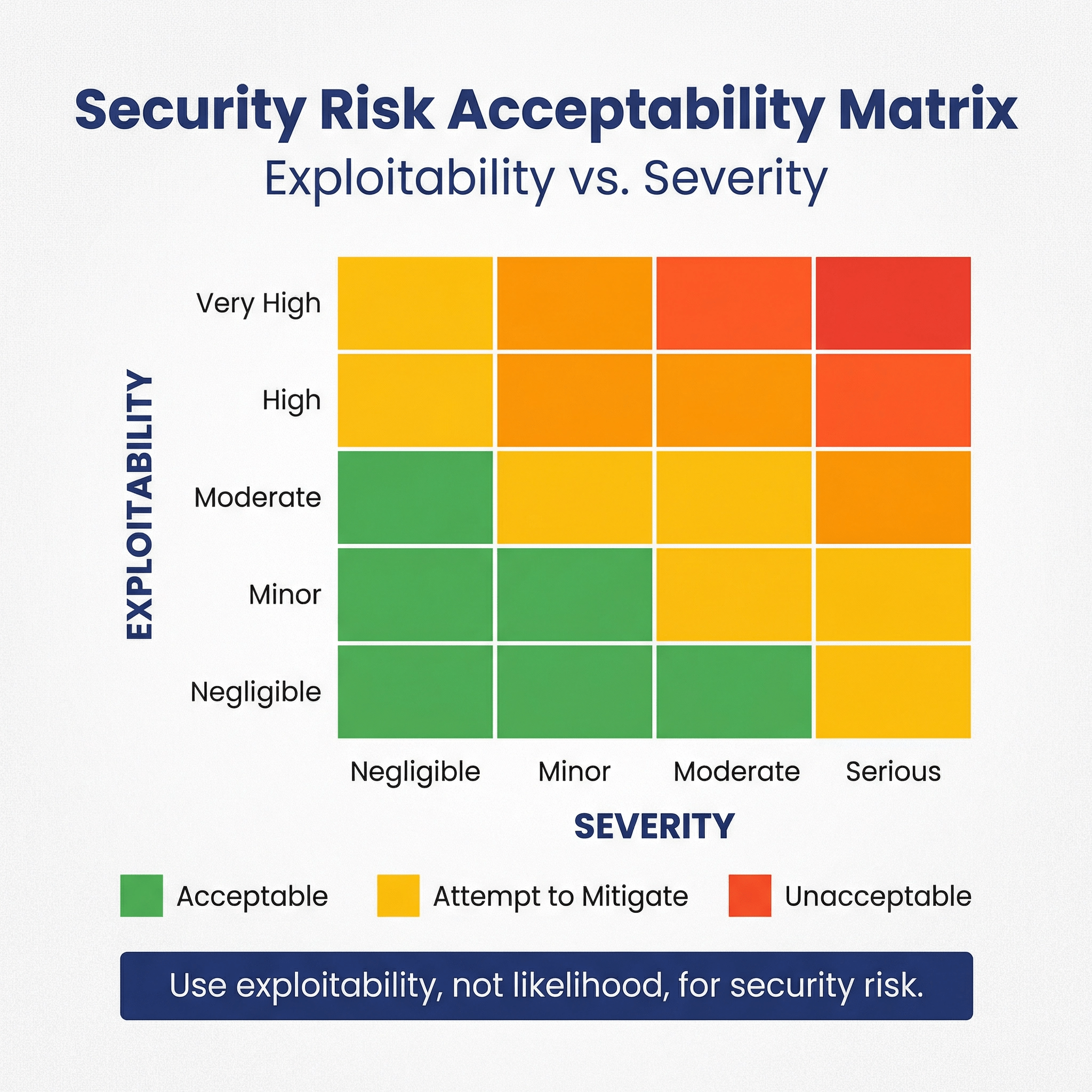
| Exploitability \ Severity | Negligible (1) | Minor(2) | Moderate (3) | Serious (4) |
|---|---|---|---|---|
| Very High (5) | Acceptable | Attempt to Mitigate | Unacceptable | Unacceptable |
| High (4) | Acceptable | Attempt to Mitigate | Attempt to Mitigate | Unacceptable |
| Moderate (3) | Acceptable | Acceptable | Attempt to Mitigate | Attempt to Mitigate |
| Minor (2) | Acceptable | Acceptable | Acceptable | Attempt to Mitigate |
| Negligible (1) | Acceptable | Acceptable | Acceptable | Attempt to Mitigate |
Security Architecture Views 🔗
What are security architecture views? 🔗
These are diagrams that show the components of the medical device system and the interfaces between these elements.
We use these diagrams as an input to the threat modeling, along with an asset inventory and connection inventory.
What is an asset in this context? 🔗
FDA defines an “asset” as “anything that has value to an individual or an organization.” This is a very broad definition. In the context of threat modeling, we primarily consider the assets called out in Appendix B of the FDA Guidance:
- Device hardware itself (including assessments for any commercial platforms)
- Applications, hardware, and/or other supporting assets that directly interact with the targeted device, such as configuration, installation/upgrade, and data transfer applications
- Healthcare facility-operated assets
- Communications/networking assets, and
- Manufacturer-controlled assets, including any servers that interact with external entities (e.g., a service that collects and redistributes device data, or a firmware update server).
More broadly speaking assets could include things like reports, images, PHI, databases, user account information, log files, security keys, backups, source code, configuration files, update files, network communication, intellectual property, or company reputation.
What is an asset inventory? 🔗
An asset inventory is a table or list of all of the assets, or system items, that make up your medical device system, along with relevant properties of those assets. It is important to have an asset inventory because there is too much information to show it all in the diagrams. We thus put most of the information in the asset inventory and include a unique ID for each asset. We then refer to these IDs within the diagrams.
What is a connection inventory? 🔗
A connection inventory is similar to the asset inventory, but its for the connections between the assets. Just as with assets, we use IDs for each connection.
Note that the uses the term “electronic interface”, while in the cybersecurity guidance uses the term “communication pathway.” The two terms are very similar, we therefore attempt to capture the required information for both in a single table.
What information do you need to capture regarding connections? 🔗
The information captured here is meant to cover the requirements in Appendix 2 (B) of the FDA Guidance as well as the interoperability questions for “electronic interfaces” in the eSTAR.
The list is quite long, and many items don’t apply in all cases. Furthermore, FDA does not typically require all of this detail, thus, we focus on including the information that we feel is useful for threat modeling.
Here’s how we like to organize the requirements from the FDA guidance into multiple properties:
-
ID
- This is a unique ID for the connection.
- It’s used to precisely link diagram elements (or explanatory text), associated hazards and controls, and testing;
-
Inactive?
- Yes/No. This is for the Interoperability eSTAR questions.
- Is the electronic interface inactive (i.e. not meant to connect, exchange, or use data with or
from other medical devices, products, technologies, or systems)?
-
Support?
- Yes/No. This is for the Interoperability eSTAR questions.
- Are the interfaces only meant for service or maintenance?
-
MDDS?
- Yes/No. This is for the Interoperability eSTAR questions.
- Is the data flow only meant for transferring, storing, converting formats, or displaying clinical
laboratory test and not intended to interpret or analyze clinical laboratory test or other device
data, results, and findings?
-
Description
- An indication of whether the path is used for data, code, and/or commands, and type of data/information/code being transferred;
- Detailed descriptions of the primary and all available functionality for each medical device system asset, including assessment of any functionality that is built in but not currently used or enabled (e.g., dormant application functionality or ports), including assurance that this functionality cannot be activated and/or misused;
- Explanations of intended behavior in unusual/erroneous/unexpected circumstances (e.g., termination of a connection in the middle of a data transfer);
- (Rare) Explanations or links to the evidence that may be used to justify security claims and any assumptions; and
-
Authentication
- Authentication mechanism (if any), including the algorithm name/version (if available), “strength” indicators (e.g., key bit length, number of computational rounds) and mode of operation (if applicable);
- Descriptions of the cryptographic method used and the type and level of cryptographic key usage and their style of use throughout the medical device system (e.g., one-time use, key length, the standard employed, symmetric or otherwise). Descriptions should also include details of cryptographic protection for firmware and software updates;
- For each authenticator created, a list of where it is verified, and how verification credentials (e.g., certificates, asymmetric keys, or shared keys) are distributed to both endpoints;
- A precise, detailed list of how each type of credential (e.g., password, key) is generated, stored, configured, transferred, and maintained, including both manufacturer- and healthcare facility-controlled assets (e.g., key management and public key infrastructure (PKI));
-
Authorization
- Access control models or features (if any) for every asset (such as privileges, user accounts/groups, passwords);
- Users’ roles and levels of responsibility if they interact with the assets and communication channels;
- Identity management (if any), including how identities are managed/transferred and configured (e.g., from manufacturer to programmer and from programmer to device);
- (Rare) Include any security configuration settings and their default values;
- (Rare) Detailed analyses by cryptography experts if a cryptography algorithm is proprietary, or a proprietary modification of a standard algorithm;
-
Communication Protocol
- Protocol name(s), version number(s), and ports/channels/frequencies;
- Any “handoff” sequences from one communication path to another (e.g., from asset to asset, network to network, or Bluetooth to Wi-Fi), and how the data, code, and/or commands are secured/protected during handoff (i.e., how is their integrity/authenticity assured);
- If communication sessions are used or supported, a detailed explanation of how sessions are established, maintained, and broken down, including but not limited to assurances of security properties such as uniqueness, unpredictability, time-stamping, and verification of session identifiers;
The examples section shows a filled in table.
Should you include all internal connections in your connection inventory? 🔗
We don’t always show internal communication if it doesn’t feel useful for the threat model.
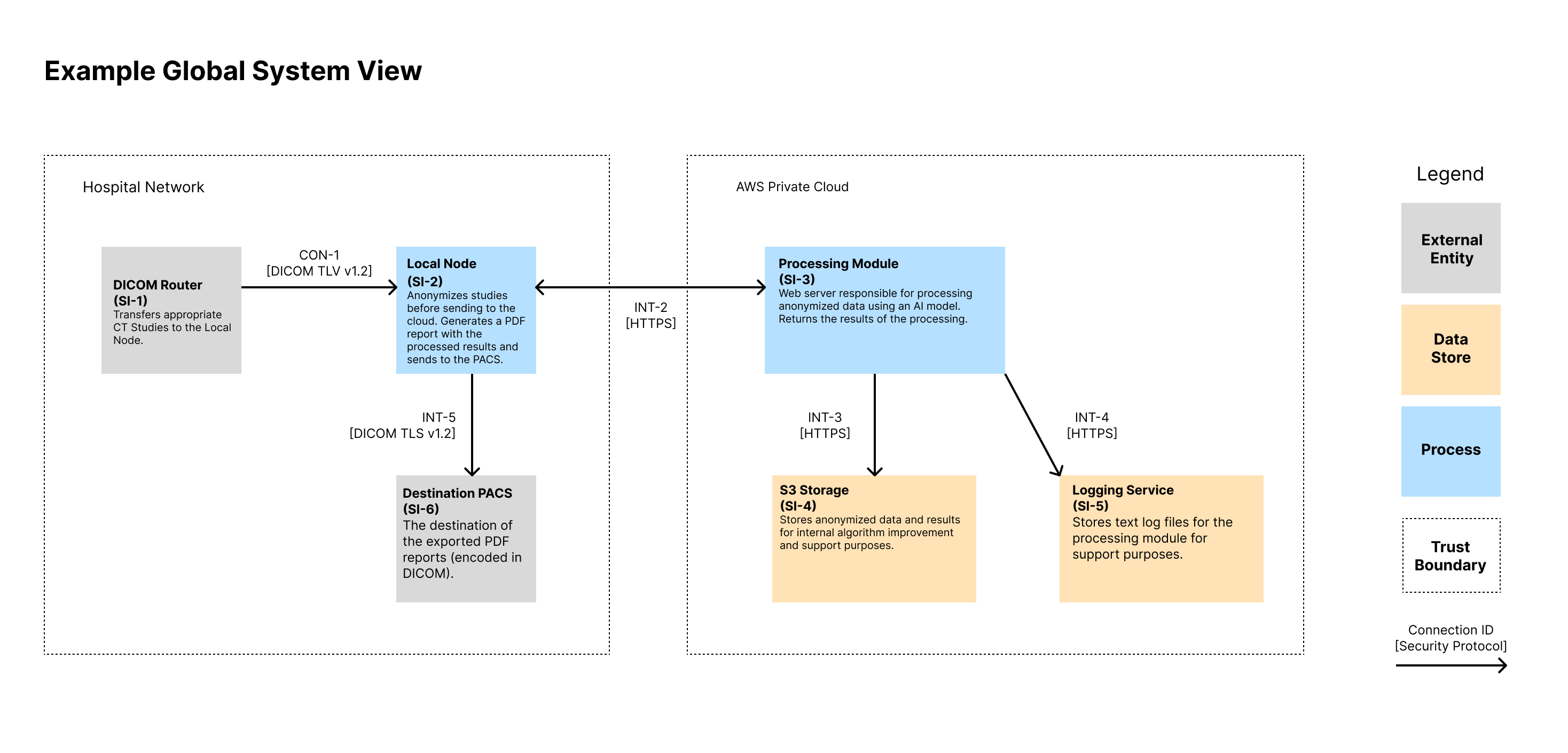
What is a global system view? 🔗
A global system view should describe the overall medical device system, including the device itself and all internal and external connections. For interconnected and networked devices, this view should identify all interconnected elements, including any software update infrastructure(s), healthcare facility network impacts, intermediary connections or devices, cloud connections, patient home network impact.
What is a multi-patient harm view? 🔗
The concept of “multi-patient harm” is introduced in the 2023 FDA Cybersecurity Guidance.
When devices are capable of connecting (wired or wirelessly) to another medical or non-medical product, to a network, or to the Internet, there is the possibility that multiple devices can be compromised simultaneously. Because of that connectivity, if a device is compromised, the device may introduce a safety risk to patients through security risk.
We’ve generally restricted “multi-patient harm” to mean a cybersecurity incident where multiple patients are harmed “simultaneously or in rapid succession”.
A multi-patient harm view demonstrates what security controls are in place to protect against multi-patient harm. E.g., it could be a sequence diagram showing the relevant connections and the location of controls.
What are some examples of multi-patient harm? 🔗
These examples are somewhat simplified, so they don’t provide all the details needed to make a firm conclusion either way. Still, I hope they’re helpful:
Cloud-Based Semi-Automated Diagnostic Radiology SaMD - This software is unlikely to cause multi-patient harm. The software does not directly interact with patients in anyway. It also isn’t on a network with other devices that directly interact with patients. Radiologists interact with the software to produce its outputs in a semi-automated way. This human-in-the loop slows down the effects of most exploits. Even if the software were fully automated or an exploit could modify the reports, there are still other clinicians downstream from the radiology report who introduce checks and delays. Thus, there wouldn’t be feasible for patients to be harmed simultaneous or in rapid succession.
Multiparameter Bedside Monitors - This example is taken from the FDA guidance. Multiparameter bedside monitors would be capable of causing multi-patient harm. They aren’t able to directly harm the patient, however, a cybersecurity attack could restart all of the monitors at once. This would leave all monitors connected to the same network no longer monitoring patient vitals and it is quite possible there wouldn’t be enough staff to monitor all patient vitals.
What is an updatability and patchability view? 🔗
An updatability and patchability view is a diagram that shows how software updates are delivered to the various parts of the system.
We usually use sequence diagrams.
What are security use case views? 🔗
Security use case views demonstrate the behavior of your system in sequence diagrams. Here are some examples of possible use cases:
- Typical clinical workflows
- Various installations or configurations (e.g., if you support on-prem or cloud installations)
- Different authentication schemes (e.g., if you support SSO for some clients)
The number and detail of your use case views depends on the security risk of your device.
Here is an example security use case view:
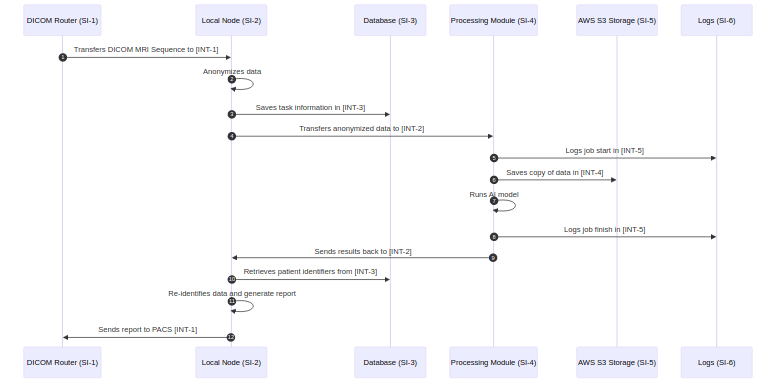
What are best practices for creating use-case views? 🔗
- Use IDs (e.g., SI-2) to unambiguously identify your system items with other diagrams documentation.
- Use connection Ids (e.g., INT-2).
- Write each arrow’s label so that it reads like a sentence when combined with the origin and destination. E.g., the first arrow can be read as “DICOM Router Transfers DICOM MRI Sequence to Local Node”.
- Don’t try to include every possible detail; only include details that are useful for helping with the threat model.
- If you exclude certain details consistently (e.g., only showing HTTP requests but not responses) note this in the document.
- Split up diagrams if there is a lot of repeat steps across different diagrams.
Threat Modeling 🔗
Do I need to create a threat model? 🔗
Yes, if your device is a cyber device, you will need to create a threat model for your FDA submission. See the threat modeling section of the 2023 cybersecurity guidance.
What is a threat? 🔗
The FDA defines a threat as follows:
“A threat is any circumstance or event with the potential to adversely impact the device, organizational operations (including mission, functions, image, or reputation), organizational assets, individuals, or other organizations through an information system via
- unauthorized access,
- destruction,
- disclosure,
- modification of information,
- and/or denial of service.”
What is threat modeling? 🔗
The FDA’s 2023 cybersecurity guidance defines threat modeling as follows:
“Threat modeling is a methodology for optimizing system, product, network, application, and connection security by identifying objectives and vulnerabilities, and then defining countermeasures to prevent, or mitigate the effects of, threats to the system.”
(Note that the 2016 post-market guidance has a slightly different definition.)
The output of the threat modeling process is a threat model.
What is typically included in a threat model? 🔗
There are many different ways of organizing and structuring threat models. Many of the approaches come from other industries. Here is the content we typically in include in our threat models which standardizes on the terminology found in the FDA guidance:
- Scope of the threat model
- Rationale for the approach used
- Who was involved in the threat modeling
- Assumptions about the system and environment of use
- An inventory of security assets
- An inventory of connections
- An inventory of expected threat sources
- Architectural diagrams including
- The key modules and layers of your device
- All external systems
- Their connections
- The process by which software updates go from source code to deployment
- An analysis of the main threats (often enumerated following a few methodologies, such as STRIDE)
- A listing of the implemented mitigations
There’s not a standard format for threat models. We will provide examples of how we format our threat models below.
What should be the scope of the threat model? 🔗
The FDA emphasizes that threat modeling must account for the entire operating environment of the device. This raises a critical question: How extensively should we consider the surroundings of the medical device? It is not always an easy question:
- For devices sold to multiple institutions, the potential scenarios are only limited by your imagination.
- For devices used within a specific lab or institution, you may wonder how far into the surrounding IT infrastructure you need to go.
We haven’t found any heuristics to offer to answer this question, but in general we are guided by the exploitability and severity of the security risks.
Which threat modeling methodologies do you suggest using? 🔗
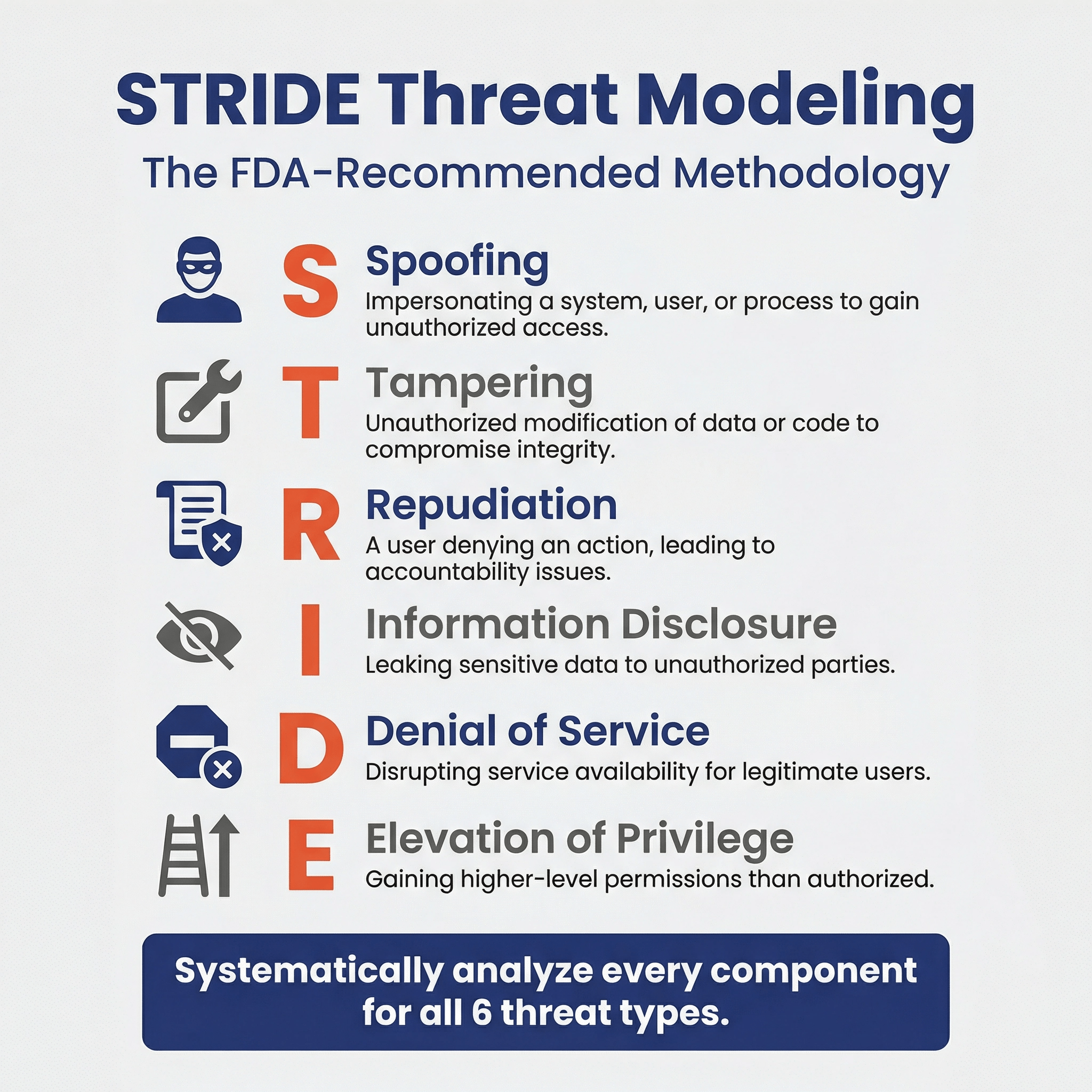
The FDA says the “rationale for the methodology(ies) selected should be provided with the threat modeling documentation.”
We suggest following the STRIDE or STRIDE and Attack Trees method for doing threat modeling. This is because the FDA-sponsored Threat Modeling Handbook mentions these methodologies, and thus, when justifying the threat-modeling methodology in your security management plan (which the FDA requires you to do), you can say that these were the methods suggested in the FDA's Threat Modeling Handbook.
How do you create a threat model? 🔗
Here is an example procedure to follow to create a threat model for an FDA submission using the STRIDE methodology.
-
Identify all major system items
- Unless your system is very simple, you often need to make judgement calls regarding what level of detail to decompose your system into. Too much detail isn’t useful.
- Threat models should encompass the full end-to-end system the device operates within, not just the device itself.
- We recommend labeling each system item (e.g., SI-1, SI-2).
- Examples: Backend Django App, AWS S3 Bucket, PACS, PostgreSQL Database, etc.
-
Create a system diagram, including connection
- These diagrams should show all the connections to external systems or networks.
- We recommend labeling each system item and connection within these diagrams.
- Create the security architecture diagrams described in the FDA guidance:
- Global System Views
- Multi-Patient Harm View (when applicable)
- Updatability and Patchability View
- Security Use Case Views (often multiple)
-
Identify and assess cybersecurity assets
- Common assets include PHI, data stores, log files, security keys, access to other systems, business reputation, etc.
- Examples of assets include PHI, AWS credentials, configuration files, PostgreSQL Database, and more abstract things like business reputation.
- We generally will indicate which system items the assets are stored within (sometimes the system items are themselves the assets).
- Once the assets are identified, we analyze the impact of the loss of confidentiality, integrity, and availability of these assets.
-
List threats for each system item using STRIDE
- Iterate through each system item and connection, reviewing the system diagrams as you go, and consider threat types according to STRIDE (Spoofing, Tampering, Repudiation, Info Disclosure, DDoS, Elevation of Privileges).
- We follow STRIDE-per-element as described in Adam Shostack’s book
- Write down these threats.
- See and for more examples.
-
Eliminate or refine threats that are highly improbable or impossible to verify
- If it is likely to impact patient safety, keep it.
- If it is unlikely to impact patient safety and is difficult to verify, consider removing it.
- If you’ve already mitigated it and it is easy to verify, keep it.
-
Trace threats to security risks and their mitigations
- Example:
- For the threat/mitigation pair
- Threat: Malicious actor brute forces the clinician’s username and password.
- Mitigation: Temporarily block login after 5 incorrect attempts.
- You might have to create a new requirement to implement the threat mitigation
- REQ-035: Throttle User Authentication
- And add a new risk to cover the threat
- SRSK-025: Brute Force Attack
- For the threat/mitigation pair
- Example:
Can you give examples of threats for each STRIDE category? 🔗
-
Spoofing: Pretending to be something or someone you’re not.
- Heuristic: What could a threat actor do if they pretended to be this element of the system?
- Example:
- Threat: Malicious actor brute forces the clinician’s username and password.
- Mitigation: Temporarily block login after 5 incorrect attempts.
-
Tampering: Changing something in a malicious way (on the disk, db)
- Heuristic: What ways could a threat actor tamper with code or data in this element?
- Example:
- Threat: Malicious actor modifies PostgreSQL Database via SQL injection.
- Mitigation: Implement input validation on frontend and backend.
- Real Insulin Pump Example:
- Background: This example is taken from the first FDA recall due to cybersecurity.
- Threat: Due to a software vulnerability, threat actors could remotely tamper with the insulin pump’s configuration via wifi.
- Mitigation: Ensure all connections to the software are properly authenticated.
-
Repudiation: Doing something bad and claiming to not have done it
- Heuristic: What actions could this element do that we want to be sure we log?
- Example:
- Threat: Malicious actor denies that a certain treatment has been prescribed.
- Mitigation: Track all prescription actions in centralized logging system.
- Drug Dispenser Example:
- Threat: A IT-person steals an opioid from a drug dispenser and modifies the logs to remove a trail of them having done so.
- Mitigation: Log important events and secure and back up the logs.
- Malpractice Example:
- Threat: A clinician makes a medical mistake; they’re worried about a lawsuit and try to cover up the mistake.
- Mitigation: Log important events and secure and back up the logs.
-
Information Disclosure: Having sensitive data leaked
- Heuristic: What are mechanisms through which data in this element could be leaked?
- Example:
- Threat: Malicious actor performs man-in-the-middle attack and steals data.
- Mitigation: Use encryption in transit (HTTPS).
-
Denial of Service: Removing availability of the system
- Heuristic: What could a threat actor do to make this element no longer available?
- Example:
- Threat: Malicious actor overwhelms backend with abnormal traffic.
- Mitigation: Configure AWS Web Application Firewall for common exploits.
- Malware Example:
- Background: This is one of the most common and pervasive threats to the HDOs.
-
Elevation of Privileges: Gaining unauthorized access
- Heuristic: What levels of authorization exist on this device? If multiple, how could an actor elevate their level?
- Example:
- Threat: Malicious user changes their role to admin via API.
- Mitigation: Only allow admins to change user roles and enforce this.
Why do you need to trace the threats to security risks? 🔗
The main benefit of threat modeling is that it is a structured process. For example, if you’re following STRIDE, you’d analyze all six types of threats to each component of the system. By systematically analyzing each component, you can be sure you didn’t miss anything. Also, by recording this analysis, engineers working on future updates to the project will understand the thought process that was used.
The security risks, on the other hand, are typically higher level. You’ll have more entries in a STRIDE threat model table than you will in your security risks. (This is similar to how an FMEA will analyze each component of the system for failure modes, and will often have more detail than the corresponding safety risks.) That said, FDA says clearly in their guidance that the threat model must “identify medical device system risks and mitigations as well as inform the pre- and post-mitigation risks considered as part of the cybersecurity risk assessment.” We take this to mean the identified threats should trace to the higher level risks, their pre- and post-mitigation impacts and likelihood levels.
When should threat modeling be done? 🔗
I like to do an initial threat model once the system architecture is reasonably well defined. (Within our software development process, this is at the end of Phase 1.) This is a good time to do threat modeling, since it will reveal design vulnerabilities in your architecture that need to be addressed by appropriate cybersecurity controls.
We then review the threat model at least once more towards the end of the development phase.
Finally, period reviews of the threat model should be performed in the post-market phase or as part of the development of new releases.
Who should create the threat model? 🔗
Software engineers who are familiar with the system should be able to perform the threat modeling, although having a cybersecurity SME involved in the process is very helpful.
Unlike with penetration testing, where we do suggest using an external firm, we don’t typically suggest having an outside firm independently put together a threat model. This is because the security risks are tightly integrated with design controls and safety risk management, and thus an external group will typically have a hard time incorporating their findings into the rest of the documentation unless they’re working closely with your team.
Having a security expert who knows the current threat landscape for your intended use environment is also helpful.
How is threat modeling related to security risk management? 🔗
The distinction between threat modeling and security risk management is vague. We suspect this is because threat modeling was developed outside the medical device industry and had its own terminology developed apart from of ISO 14971. In any event, it is straightforward to map the components of the Threat Modeling Manifesto to the AAMI TIR57 security risk management activities, as shown in the following diagram.
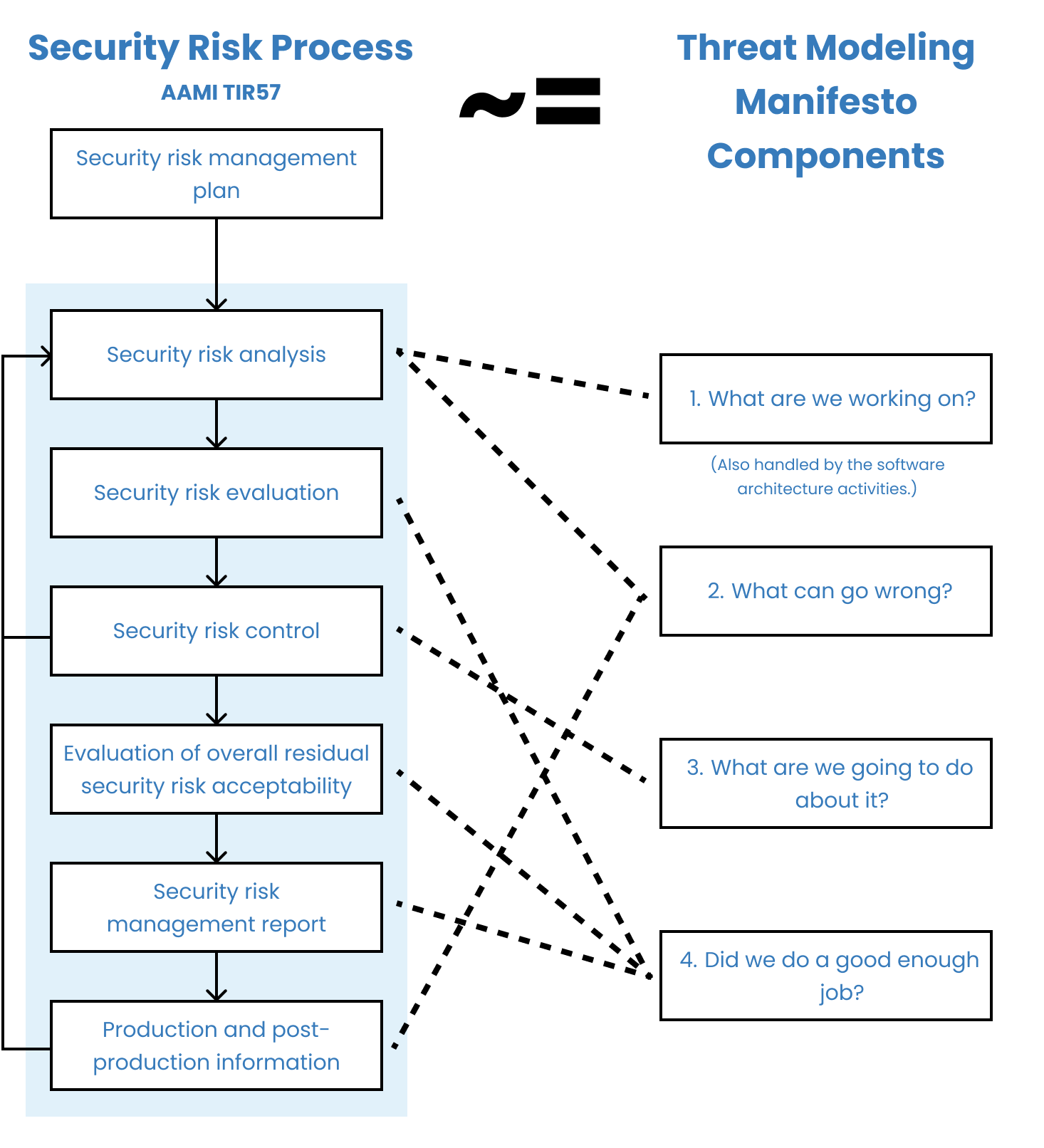
If we were to make a distinction, we would say that “threat modeling is a loose collection of methodologies used to systematically identify, mitigate, and document security risks.”
According to this distinction, threat modeling is to security risk management as FMEA is to safety risk management. FMEA is a structured way of identifying safety risks related to parts of your system breaking down. Unlike FMEA, which is rarely sufficient to identify all of the safety risks for a device because it misses process- and usability-related risks, threat modeling is in theory sufficient on its own. Thus, even this attempt at a distinction isn’t very useful.
What other resources exist for threat models? 🔗
Here are several articles:
- Promenade’s guide to their hybrid asset and vulnerability based threat modeling
- Adam Shostack’s Threat Modeling Book
- Threat Modeling Manifesto
- OWASP Threat Modeling Cheat Sheet
- The Ultimate List of STRIDE Threat Examples
-
MITRE’s “Playbook for Threat Modeling Medical Devices”
- Note that this playbook is now out-dated and doesn’t align with the latest FDA guidance.
Here are a few tools that are widely used:
- Microsoft Threat Modeling Tool (uses STRIDE per Element; Windows Only)
- OWASP Threat Dragon (more flexible; doesn’t generate threats; multi-platform)
Cybersecurity Testing Overview 🔗
What types of cybersecurity testing does FDA require? 🔗
FDA suggests considering the following four types of cybersecurity testing:
| Testing Type | Description |
|---|---|
| Security Requirements Testing | This is normal design verification for all of your cybersecurity-related software requirements. |
| Threat Mitigation Testing | This is normal design verification for the risk control measures identified in your threat modeling and security risk assessment. |
| Vulnerability Testing | Vulnerability testing is a type of cybersecurity testing that focuses on identifying and characterizing security vulnerabilities. |
| Penetration Testing | A penetration test (pen test) is an authorized simulated attack performed on a medical-device system to evaluate its security. |
Vulnerability Testing 🔗
What is Vulnerability Testing? 🔗
Vulnerability testing is a type of cybersecurity testing that focuses on identifying and characterizing security vulnerabilities. A vulnerability is a “a weakness in an information system, system security procedure(s), internal control(s), human behavior, or implementation that could be exploited.”
Do all cyber devices need vulnerability testing? 🔗
Yes! Unlike some of the other cybersecurity testing activities, the need for vulnerability testing is actually included in the legal statute itself, which says cyber devices must:
What are some specific types of vulnerability testing we should do? 🔗
In the 2023 FDA guidance, the following types of testing are suggested for consideration:
| Type | Example |
|---|---|
| Abuse or misuse cases, malformed and unexpected inputs | Entering special characters or extremely long strings into input fields |
| Robustness testing | Stress testing the system under high load or with limited resources |
| Fuzz testing | Automatically generating and submitting random data to API endpoints |
| Attack surface analysis | Identifying all possible entry points for attacks, such as open ports or APIs |
| Vulnerability chaining | Combining multiple low-severity vulnerabilities to create a high-severity exploit |
| Closed box testing of known vulnerability scanning | Using automated tools to scan for known vulnerabilities without prior knowledge of the system |
| Software composition analysis of binary executable files | Analyzing compiled code to identify potentially vulnerable third-party libraries |
How should we document the results of our Vulnerability Testing? 🔗
Per the FDA guidance, for any testing tools or software used, the details provided may include, but may not be limited to, the name of the tool, version information as applicable, and any settings or configuration options for the tools used.
Fuzz Testing 🔗
What is fuzz testing? 🔗
Fuzz testing, also sometimes called fuzzing, is a particular type of vulnerability testing technique. Simply put, it involves feeding randomized inputs to the software and seeing if anything breaks.
Do we have to do fuzz testing? 🔗
FDA recommends that “fuzz testing” be considered for submission in their 2023 FDA Guidance. We’ve had mixed luck pushing back on the need for fuzz testing with FDA. In several submissions, FDA has accepted that fuzz testing was not suitable. On others, FDA has held their ground.
Traditionally, fuzz testing has focused on languages like C or C++ which are compiled and prone to memory errors, however, fuzzing can be applied to software built with interpreted languages such as JavaScript and Python too.
What attack surface should we use for our fuzz testing? 🔗
There are several different “attack surfaces” that could be fuzz-tested. At the lowest level, you can fuzz test your individual functions or classes. With regards to cybersecurity fuzz testing, the external interface of your device is most appropriate.
See Part 1 section 15 of UL-2900 Software Cybersecurity for Network-Connectable Products for some more structured details about “malformed input testing” (of which fuzz testing is a type). It says “The product shall continue to operate as intended when subject to invalid or unexpected inputs on its external interfaces.”
How much fuzz testing is enough? 🔗
Since fuzz testing involves feeding random inputs into an interface or API, there is always the question of “how much is enough”? There’s no clear answer here, but this is something your test reports that will be in your 510(k) submission would ideally address.
The UL-2900 standard says:
This may be a good starting place.
Can we rely on the fuzzing our pen tester did? 🔗
External pen testers may perform fuzz testing as part of their pen testing suite. If they do, you may be able to “take credit” for the fuzz testing they already did. If you plan ahead for this, it would be good to have information about the interfaces they tested, how long they fuzzed, and how many inputs they fed into the tests in the final report they produce.
Where can I learn more about Fuzzing? 🔗
Here are some good resources:
- Section 15 of the UL-2900-1 standard (costs money to purchase) has some requirements that may help build out a structure for a fuzz testing procedure.
- This Synopsys article has a great technical intro to fuzz testing.
- This OWASP page is semi-useful and has some links.
Penetration Testing 🔗
What is penetration testing? 🔗
A penetration test (pen test) is an authorized simulated attack performed on a medical-device system to evaluate its security. Penetration testers act as if they were attackers to find and demonstrate the business impacts of weaknesses in a device. Penetration tests usually simulate a variety of attacks that could threaten a device, using the same tools, techniques, and processes that real hackers use.
What is pentesting? 🔗
Pentesting is shorthand for penetration testing. We usually use the longer form because this is what the FDA uses in their guidance and in our documentation we generally try to follow the FDA’s lingo to make it easier on reviewers to follow our submissions.
How is penetration testing different from vulnerability testing? 🔗
Vulnerability testing focuses on identifying and characterizing vulnerabilities. Penetration testing involves trying to discover and exploit vulnerabilities and other weaknesses in the system. Thus, vulnerability testing will stop once the vulnerabilities are identified. Penetration testing will take the next step and will try to actually exploit the vulnerability.
Thus, penetration testing has fewer false positives than vulnerability testing, which often finds many vulnerabilities that can’t actually be exploited in practice.
Do all cyber devices need penetration testing? 🔗
Most, but not all. Unlike other regulatory requirements, such as including an SBOM, FDA doesn’t require penetration testing in every case.
In most cases we strongly suggest doing pen-testing.
We usually include pen testing in our submissions, but we have had a couple of devices with very limited threat surfaces get FDA-cleared without pen testing.
Can we do the penetration testing ourselves? 🔗
FDA suggests that the penetration testing have a level of independence from the team that developed the software. The best way to accomplish this is to use a separate firm.
We have had a few clients successfully use someone else on their team to do pen-testing, however it is important that:
- The person was not involved in the development of the application
- The person has credentials and certifications for doing pen-testing
FDA will almost certainly not allow an engineer who helped develop the device do the testing. They also won’t be content with someone who just picked up some pen-testing tools online and ran them against the software.
How do you scope penetration testing? 🔗
Here are some questions to ask yourself about this:
- What connections are being tested? E.g., will you be testing blue-tooth connections, USB ports, cloud infrastructure, your servers, etc.
- Will you pen-test the device’s update process?
- For SiMD, will you need to ship the device to the client or will the pen-testers need to fly on site?
- How much information about the device will you share with the pen-testers? Will it be black-box, gray-box, or white-box testing?
- Will you want the vendor to re-run the pen-tests after your team has had a chance to address issues?
How should we evaluate vendors to do our penetration testing? 🔗
Once you’ve identified the scope of your pen testing, you can share this with vendors to see if they’re a good fit and if they’ll provide you a quote.
Here are some questions you may want to ask:
- If you have SaMD, make sure they do application penetration testing (and not network IT infrastructure pen testing).
- If you have a physical device, make sure they do device penetration testing.
- How much lead time do you need before you can do pen testing?
- This is important, since ideally you’ll do pen testing just before your submission and you don’t want a big delay to be a bottleneck.
- Can you provide us a sample report?
- You will want to check that the testing report will include the following elements required by FDA:
- Independence and technical expertise of testers;
- Scope of testing;
- Duration of testing;
- Testing methods employed; and
- Test results, findings, and observations.
- You will want to check that the testing report will include the following elements required by FDA:
- Do you have discounts for doing repeated tests?
- Keep in mind you’ll likely be repeating the penetrating testing (e.g., annually), so it’s often helpful to have a single vendor you use.
Should I select pen-testers that focus on medical-devices? 🔗
If you need help with threat modeling or security risk management, it can be helpful to have a pen-tester who knows the FDA regulations. In general though, we don’t believe it is necessary to work with pen-testers who focus just on medical-devices. We’ve had several submissions with pen-testers who do not focus on the medical device sector.
How much does penetration testing cost? 🔗
It varies from vendor to vendor and based on the scope of the testing, but you can expect between $7k and $22k for basic testing of a web-only system. The price increases with the number of connections and get quite high—even as high as $200k in some instances.
When should we do our penetration testing for a premarket submission? 🔗
Ideally, your penetrating testing will be done on the final version of the software that you’re submitting to FDA. This is because FDA recognizes that the results of penetration testing degrade over time. FDA will likely accept pen-tests that have been completed within a year of the submission, however, older than that likely will not be acceptable. Furthermore, you’ll need to justify why any changes that have been made to the software between the pen test and the submission don’t affect the cybersecurity. E.g., if you change how authentication is processed or add new user roles, you would likely need to do another round of pen-testing.
How often should we do penetrating testing? 🔗
We suggest documenting in your Security Management Plan when and how often you plan to do penetration testing. In the FDA guidance, they suggest repeating it periodically:
We generally suggest that if you need penetrating testing that you repeat it annually. That said, this would most likely be a finding in an audit, and wouldn’t affect your pre-market submissions.
How to Learn More 🔗
What FDA guidance apply to medical-device cybersecurity? 🔗
There are two essential FDA guidance documents that apply to medical devices:
- 2023 FDA Guidance - Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions This guidance is a good place to start.
- 2016 FDA Guidance - Postmarket Management of Cybersecurity in Medical Devices This guidance goes into more detail about post-market surveillance, but there is overlap between the two.
There is also a short, older guidance about cybersecurity of OTS software.
What standards apply to medical-device cybersecurity? 🔗
The FDA has recognized several standards (search the FDA database using the “security” keyword and you can see most of them). Of all of these, we feel that IEC 81001-5-1:2021 Health software and health IT systems safety, effectiveness and security — Part 5-1: Security — Activities in the product life cycle is the most important. It defines the activities you need to follow to build a secure device, similar to how IEC 62304 defines the activities you need to follow to build a safe device. The FDA suggests following a standard such as this in their 2023 guidance.
Examples 🔗
Threat Model of a Common Radiology AI Deployment 🔗
Overview 🔗
This example is of a common architecture we see with our AI-enabled radiology software clients. It involves two primary software components. One sits within the hospitals network and is responsible for receiving medical images, anonymizing them, and forwarding them along to the cloud for processing. The cloud component processes the data, stores a copy online for the company to review and assess, and sends the results back to the local node. There, the local node generates a report
Asset Inventory 🔗
| ID | Name | Type | Description |
|---|---|---|---|
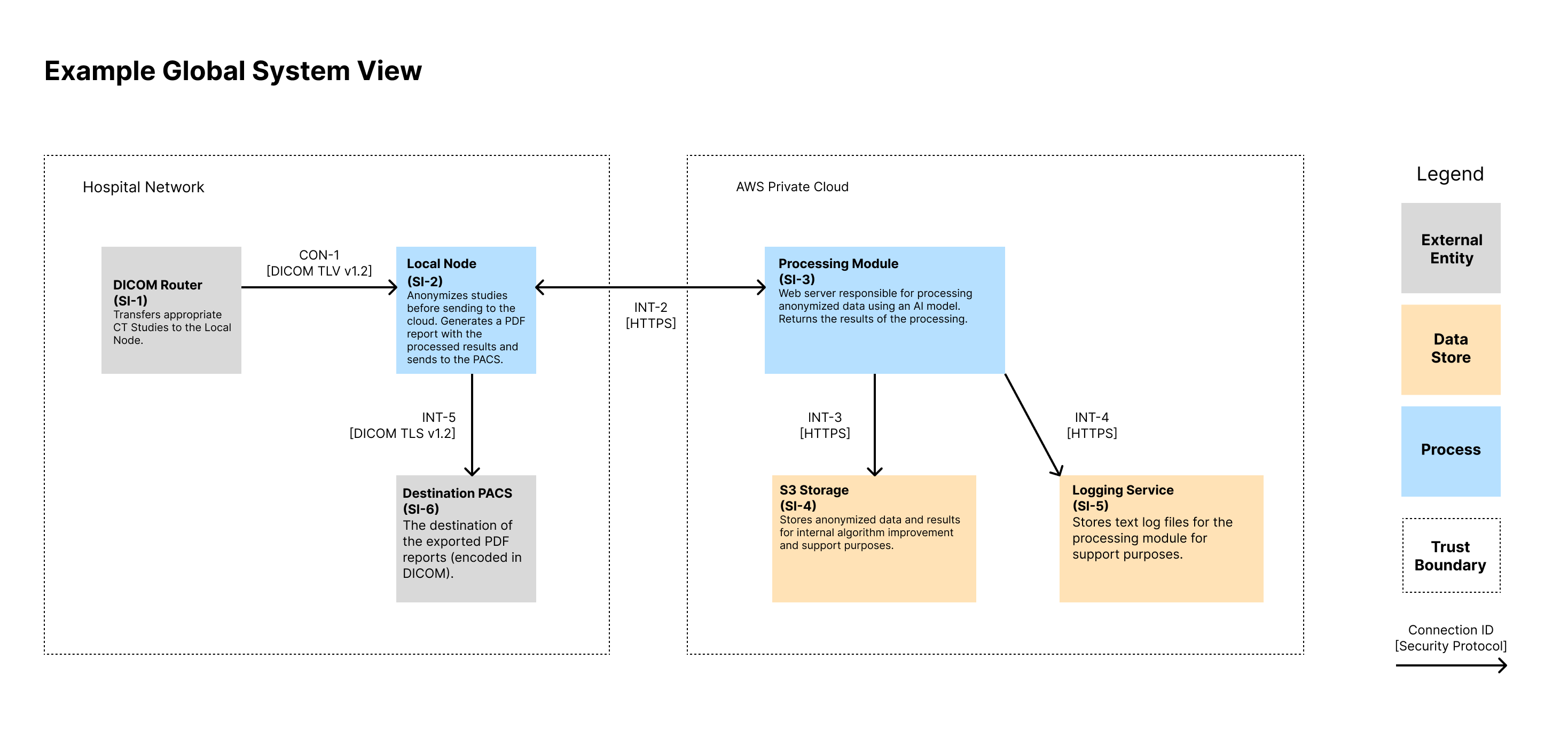
| SI-1 | DICOM Router | External Entity | Source of inputs to be processed |
| SI-2 | Local Node | Process | Local Docker Image that lives in the hospital network. It anonymizes inbound CT Studies before sending to the cloud for processing. Upon receiving a response, it generates a PDF report with the processed results and sends to the PACS. It includes a local database for tracking ongoing tasks. |
| SI-3 | Processing Module | Process | Web server responsible for processing anonymized data using an AI model. Returns the results of the processing. |
| SI-4 | S3 Storage | Data Store | Stores anonymized data and results for internal algorithm improvement and support purposes. |
| SI-5 | Logging Service | Data Store | Stores text log files for the processing module for support purposes. |
| SI-6 | Destination PACS | Data Store | Destination of device outputs |
Connection Inventory 🔗
| ID | Inactive? | Support Only? | MDDS? | Description | Authentication | Authorization | Communication Protocol |
|---|---|---|---|---|---|---|---|
| INT-1 | No | No | No | Used to transfer DICOM CT Studies for processing. | Both systems authenticate using X.509 certificates. The Local Node’s CSR and keys are generated at installation and maintained by the customer. | Authorization is implicit via mutual TLS and firewall ACLs allowing only authorized endpoints. | DICOM over TLS v1.3 on a configurable port |
| INT-2 | No | No | No | The API used by the local node to trigger a processing request. Anonymized image data and metadata is sent over the API. | The Local Node (SI-2) is authenticated by SI-3 using the OAuth 2.0 Client Credentials Flow. The client ID and secret are distributed by the manufacturer on install. Access tokens are short-lived and scoped. | Access tokens grant permission to process new inputs. No API endpoints provide read access to any information on the server. Token introspection is performed on each request. | HTTPS over TLS v1.3. JSON-based REST API |
| INT-3 | No | Yes | No | Transfers the processing inputs and outputs from SI-3 to SI-4 for support purposes. | IAM roles using short-lived credentials from metadata service. Keys are rotated every 90 days. | AWS IAM policies restrict actions to specific buckets and prefixes, enforcing least privilege. | HTTPS over TLS v1.3. JSON-based REST API |
| INT-4 | No | Yes | No | Sends application and error logs from SI-3 to SI-5. Data includes timestamps, processing metadata, and stack traces. | IAM roles using short-lived credentials from metadata service. Keys are rotated every 90 days. | IAM roles scoped to log-only operations (PUT object) for defined paths. No read/delete allowed. | HTTPS over TLS v1.3. JSON-based REST API |
| INT-5 | No | No | No | Used to transfer final PDF report to the output PACS. Transfer uses DICOM SR or PDF encapsulated in DICOM. | Both systems authenticate using X.509 certificates. The Local Node’s CSR and keys are generated at installation and maintained by the customer. | Authorization is implicit via mutual TLS and firewall ACLs allowing only authorized endpoints. | DICOM over TLS v1.3 on a configurable port |
Global System View 🔗
Note that we show the IDs for the System Items (i.e., Assets) in the diagram as well as the connection IDs. These IDs can then be traced to documentation that contains more information that could fit on the diagram. We like to show a high-level description of the assets in each node. We also like to display the protocol of each connection.
Security Use Case View 🔗
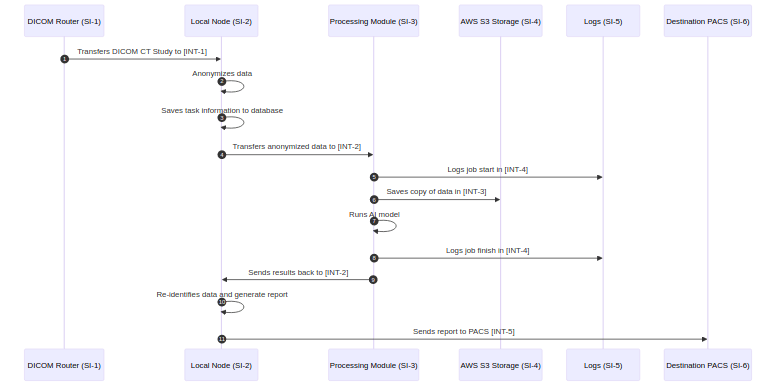
To create these sequence diagrams, I like to use mermaid. Our Medtech OS platform supports this out of the box, but you can also use their online tool tool. Here is the mermaid text that created the above diagram:
sequenceDiagram
autoNumber
participant si-1 as DICOM Router (SI-1)
participant si-2 as Local Node (SI-2)
participant si-3 as Processing Module (SI-3)
participant si-4 as AWS S3 Storage (SI-4)
participant si-5 as Logs (SI-5)
participant si-6 as Destination PACS (SI-6)
si-1->>si-2: Transfers DICOM CT Study to [INT-1]
si-2->>si-2: Anonymizes data
si-2->>si-2: Saves task information to database
si-2->>si-3: Transfers anonymized data to [INT-2]
si-3->>si-5: Logs job start in [INT-4]
si-3->>si-4: Saves copy of data in [INT-3]
si-3->>si-3: Runs AI model
si-3->>si-5: Logs job finish in [INT-4]
si-3->>si-2: Sends results back to [INT-2]
si-2->>si-2: Re-identifies data and generate report
si-2->>si-6: Sends report to PACS [INT-5]
Patchability and Updatability View 🔗
NOTE: The patchability and updatedability view is a WIP. I’d usually show system items for these as well and would display them on the global system view.
Threat Modeling - STRIDE 🔗
STRIDE Threat Modeling was completed on 2025-07-03 by the following individuals:
| Name | Organization |
|---|---|
| J. David Giese | Innolitics, LLC |
The following scope and assumptions applied to the threat modeling:
- The security architecture views are accurate and complete.
- The scope of the STRIDE threat modeling only includes the top-level System Items and Connections.
- It includes the system items that are part of the build process.
- It assumes the healthcare facilities network is inherently hostile.
- The STRIDE-per-Element methodology was used, where specific STRIDE threats are considered for each of four types of items, as shown in the table below:
| Type | Spoofing | Tampering | Repudiation | Information Disclosure | Denial of Service | Elevation of Privilege |
|---|---|---|---|---|---|---|
| External System | X | X | ||||
| Process | X | X | X | X | X | X |
| Connection | X | X | X | |||
| Data Store | X | X | X |
Here are the results of the STRIDE-per-element threat modeling, along with links to the related security risks (if any).
| Element | Type | Spoofing | Tampering | Repudiation | Information Disclosure | Denial of Service | Elevation of Privilege |
|---|---|---|---|---|---|---|---|
| SI-1 DICOM Router | External System | If someone spoofed the DICOM router, they could send you DICOM commands. Mitigations should include ensuring you limit SI-2 to accepting certain commands. Also, you’d want to ensure SI-2 isn’t susceptible to a denial of service attach | N/A | If logs of DICOM C-STORE operations are not protected, the sender could deny having transferred a particular study. | N/A | N/A | N/A |
| SI-2 Local Node | Process | Stolen OAuthclient‑credentials or TLS keys let an attacker impersonate SI‑2 toward cloud or PACS. | If an attacker alters the Python anonymization script (e.g., by injecting into the Docker volume), they could disable anonymization or insert custom metadata into the study. | Insider deletes local audit logs so it cannot be proved that a given study was processed. | If the database used accepts unauthenticated local socket connections, task data and status logs could be dumped by a local attacker. | If the Local Node accepts multiple parallel C-STORE requests without throttling, an attacker could flood it with large studies, blocking memory and CPU for legitimate jobs. | If the Local Node runs as root and exposes a vulnerable API (e.g., debug endpoint), arbitrary code execution could yield host-level access. |
| SI-3 Processing Module | Process | Spoofed DNS entry lures the LocalNode to post data to a malicious look‑alike API. | Compromised CI/CD pipeline pushes a modified AI model that silently alters diagnoses. | Cloud admin deletes request logs and claims the offending job never reached the service. | Debug responses leak stack traces and environment variables containing PHI or tokens. | Burst of bogus jobs consumes GPU quotas, delaying legitimate traffic. | If AWS roles are misconfigured with wildcard IAM permissions, remote code execution could allow access to other internal S3 buckets. |
| SI-4 S3 Storage | Data Store | N/A | Compromised IAM role overwrites or deletes stored objects, corrupting historical data. | N/A | Bucket made public‑read by mistake, leaking anonymized images & results. | Bulk‑delete or quota exhaustion makes the bucket unavailable for new writes. | N/A |
| SI-5 Logging Service | External System | If someone spoofed the logging service endpoint via DNS tampering, SI-3 would POST logs to an attacker-controlled sink. | N/A | If the Logging Service accepts log submissions over HTTPS without proper authentication, a malicious service could upload logs and later deny doing so. | N/A | N/A | N/A |
| SI-6 Destination PACS | Data Store | N/A | Malware on PACS rewrites embedded PDF reports before radiologist review. | N/A | Unencrypted backup of the PACS DB exposes all processed reports. | Flood of malformed DICOM objects fills storage and blocks new studies. | N/A |
| INT-1 | Connection | N/A | Man‑in‑the‑middle (MITM) alters DICOM header fields (e.g., StudyInstanceUID) enroute, causing mis‑routing. | N/A | Protocol‑downgrade attack forces plaintext, letting attacker capture metadata. | Flood on the configured port blocks study uploads. | N/A |
| INT-2 | Connection | N/A | MITM alters processing parameters in the JSON payload, leading to wrong algorithm results. | N/A | Compromised bearer‑token from a compromised proxy lets attacker submit jobs. | Flood of huge multi‑frame studies exceeds API rate limits, starving real traffic. | N/A |
| INT-3 | Connection | N/A | Compromised EC2 instance uses temporary creds to rewrite objects in bucket. | N/A | If AWS IAM roles are misconfigured on the bucket, data could be accessed improperly. | Upload of extremely large objects exhausts bucket quota & blocks writes. | N/A |
| INT-4 | Connection | N/A | MITM injects false log lines, polluting forensic evidence. | N/A | Intercepted log stream reveals stack traces containing PHI or secrets. | If a high-volume job triggers hundreds of thousands of log entries, the logging system may degrade or crash. | N/A |
| INT-5 | Connection | N/A | If a malicious Local Node sends DICOMs files with invalid measurements, the PACS would store and display misleading reports. | N/A | If host verification is not in place, a rogue PACS system could capture outgoing PHI. | If thousands of DICOMs are submitted in quick succession, the PACS may stall due to processing overload. | N/A |
Security Risk Assessment 🔗
| ID | Name | Sequence of Events | Leads to Safety Risks | Exploitability Pre-RCM | Severity Pre-RCM | Acceptability Pre-RCM | RCMs | Mitigations Cause | Exploitability Post-RCM | Severity Post-RCM | Acceptability Post-RCM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SRSK-1 | Wrong-patient association via header tampering | 1) Attacker MITMs SI-1→SI-2 on INT-1; 2) Alters StudyInstanceUID / patient metadata; 3) SI-2 ingests, re-IDs to wrong patient; 4) Report delivered to SI-6. | Yes | Minor | Serious | Attempt to Mitigate | R-2 DICOM TLS R-3 AE Allow-lists R-4 Study Integrity Check |
None | Negligible | Serious | Acceptable |
| SRSK-2 | Repudiation of inbound DICOM study | 1) A clinical support incident occurs (e.g., wrong patient data in report) 2) DICOM operator claims they didn’t send the problematic study. |
No | Minor | Moderate | Attempt to Mitigate | R-1 Log Input Requests | None | Negligible | Moderate | Acceptable |
| SRSK-3 | Anonymization script tampering | 1) Local attacker modifies Python anonymizer via writable volume; 2) PHI not stripped or identifiers mis-mapped; 3) Re-ID step maps to wrong patient or leaks PHI. | Yes | Minor | Serious | Attempt to Mitigate | R-5 Container Root Read-only | None | Negligible | Serious | Acceptable |
| SRSK-4 | Malicious/compromised AI model via CI/CD | 1) Supply-chain compromise pushes altered model to SI-3; 2) Inference outputs biased/incorrect; 3) Wrong content in report. | No | Minor | Moderate | Attempt to Mitigate | R-7 Code Review R-8 Controlled Model Deployment |
None | Negligible | Moderate | Acceptable |
| SRSK-5 | Token compromise leading to unauthorized job | 1) Bearer token stolen from SI-2; 2) Attacker submits crafted jobs; 3) Results get re-identified and sent to PACS. | No | Moderate | Moderate | Attempt to Mitigate | R-9 Credentials in OS Keychain R-10 Expiring Tokens |
None | Minor | Moderate | Acceptable |
| SRSK-6 | Denial of Service on SI-2 | 1) Attacker floods INT-1 with large studies/parallel requests; 2) SI-2 CPU/mem saturated; 3) Legitimate jobs delayed. | Yes | Moderate | Moderate | Attempt to Mitigate | R-6 Resource Limits | None | Negligible | Moderate | Acceptable |
| SRSK-7 | DNS spoofing of SI-3 | 1) SI-2 resolves attacker-controlled host; 2) Uploads anonymized data/results to wrong API; 3) Exfiltration or fake results. | No | Minor | Serious | Attempt to Mitigate | R-11 Authenticated & Pinned Connections | None | Negligible | Serious | Acceptable |
Security Controls 🔗
| ID | Name | Type | Requirement |
|---|---|---|---|
| R-1 | Log Input Requests | Event Detection & Logging | The SI-2 Local Node must log incoming DICOM transfers, the timestamp, and Study UID. |
| R-2 | DICOM TLS | Authentication | The system shall support DICOM communication over TLS when TLS is supported by the connected site. |
| R-3 | AE Allow-list | Authorization | The SI-2 Local Node shall accept incoming DICOM transfers only from sources whose IP addresses or VLANs are on an approved allowlist. |
| R-4 | Study Integrity Check | Code, Data and Execution Integrity | The system shall verify the identity of a received study by comparing the StudyInstanceUID and related metadata to expected values, and shall reject transfers with mismatches. |
| R-5 | Container Root Read-only | Confidentiality | The system shall mount the container root filesystem as read-only and run the container as a non-root user. |
| R-6 | Resource Limits | Resiliency and Recovery | The system shall enforce CPU and memory resource limits and isolation to prevent saturation from excessive or malicious requests |
| R-7 | Code Review | Resiliency and Recovery | All code changes shall undergo code review and approval by at least one other engineer before deployment. |
| R-8 | Controlled Model Deployment | Firmware and Software Updates | The system shall deploy models only from a controlled model registry with defined promotion gates, and shall verify deterministic model hashes before activation. |
| R-9 | Credentials in OS Keychain | Cryptography | The system shall not store client credentials on local disk; credentials shall be kept in a secure in-memory store or hardware/software key management system. |
| R-10 | Expiring Tokens | Resiliency and Recovery | The system shall use authentication tokens that automatically expire to limit the impact of credential compromise. |
| R-11 | Authenticated & Pinned Connections | Authentication | The system shall use mutual TLS and DNS hardening, including certificate pinning, for communications when supported by the connecting site. |
Revision History 🔗
| Date | Summary of Change |
|---|---|
| 4 August 2023 | Initial Version; It’s still rough around the edges, but useful enough to publish. |
| 19 December 2023 | Add more details about vulnerability testing and penetration testing, taking into account ideas from IEC 81001-5-1:2001 |
| 22 January 2024 | Add a section about fuzz testing |
| 30 June 2024 | Added more details in the threat modeling document; fix various typos; add comments about multi-patient harm; add details about evaluating security risks |
| 26 September 2024 | Add more questions about cybersecurity testing |
| 7 November 2024 | Clarified the threat modeling questions; added an example threat model |
| 24 June 2025 | Added questions about deliverables in the eSTAR. Improved and expanded on the examples. Added questions about security architecture diagrams. |
| 13 August 2025 | Expand the threat model and security risk assessment examples. |



