We are an engineering, regulatory, and management consulting firm focused exclusively on AI-enabled medical device software. We help CEOs and founders turn breakthrough clinical AI into FDA-cleared, market-ready products—with guaranteed timelines and outcomes—All under one roof.
Multi-Year Partnership: CBCT Development → FDA Clearance → Maintenance → Support → M&A Support
The client’s engineers had never touched regulatory. We got them FDA cleared on their first submission. Now they are self sufficient.
Client's first medical device. No documentation. 510(k) cleared in 12 months while engineers stayed focused on dev.
Client received hold letter with major cybersecurity findings. Engaged Innolitics. Cleared in 3 months.
Other consultants couldn't make progress. We finished their software docs in 4 months — 3x faster than expected.
Resolved FDA hold letter that would have required repeating the entire clinical study. Cleared shortly after.
Built and cleared an AI cardiomegaly detector end-to-end: algorithm R&D to 510(k) clearance in under 12 months.
Full-cycle development of GyriCalc, a first-of-its-kind pediatric neuroimaging diagnostic. FDA cleared (K250686) July 2025. Filed for IPO in June 2025.
Built and cleared a DICOM image processing SaMD in 14 months. Full-cycle: engineering to 510(k) clearance.
We built the app, trained the AI, ran the MRMC study, and cleared FDA.
FDA regulatory strategy and Pre-Submission for an AI platform converting 2D MRI to 3D joint models. Delivered on time and budget
We isolated Neosoma's new CNN for FDA review, won clearance in 3 months, and built a framework for every product after.
After spending $500K over five years on consultants who knew how to sell but couldn't execute, Innolitics took us from concept to FDA clearance in under 18 months for a first-of-its-kind MRI neuroimaging device. When FDA questioned our original approach, they adapted without losing momentum. We submitted on schedule and cleared in 4.5 months. I've seen many engineers at J&J and Ethicon — Innolitics is many standard deviations ahead of the curve.
They were proactive, informative, and very well-versed in the regulatory challenges of AI in medical imaging… Unlike competitors, who sometimes take a blanket approach, Innolitics didn't throw the baby out with the bathwater. They recognized the good work already done and built upon it… We reviewed other capable firms, but none showed the same depth of understanding of the rapidly evolving landscape of AI and regulatory requirements. Their proactive stance with the FDA gave us confidence that we were in the right hands, compared to competitors who seemed more reactive.
Working with Innolitics was a true game-changer for us. Their exceptional expertise in both engineering and regulatory affairs enabled us to speed up our product development and achieve FDA clearance within a year. Innolitics is more than just an engineering firm—they're a trusted partner. I have complete confidence in their technical skills to build things right the first time.
Innolitics are more than a group of top SaMD engineers and reg consultants—they are trusted advisors and are like family. They are a one stop shop for AI/ML algorithm R&D, full stack web development, and FDA regulatory clearance. We built SmileDx, a dental CADe from scratch all without needing to raise external funding and within a reasonable timeframe.
I am happy to have "roped in" Innolitics into our medical device journey. Their ability to break down complex technical jargon into actionable insights is incredible. I have come to trust their business, technical, and regulatory sense—and so has the FDA so far. I recommend them to anyone looking for a one stop SaMD shop.
7 weeks to submission and 6 months to FDA clearance. Innolitics delivered exactly what we needed. They took ownership of the entire DHF and 510(k) package—documentation, cybersecurity, risk management, the works. When we hit internal roadblocks, they found ways to keep us moving. When scope decisions needed to be made fast, they made the right calls. Fast, thorough, no surprises, and full service. That's how regulatory consulting should work!
Our first FDA clearance was monolithic — every component submitted as one device. When we needed to clear a second AI indication, we assumed we'd have to re-submit the entire platform. Innolitics saw something we didn't: a modular regulatory architecture that isolated the new CNN as the only component under review. That single insight saved us months of redundant documentation and positioned every future tumor type as a lightweight module swap. We submitted in September 2025 and cleared in December — 3-month review, zero major deficiencies. I've worked with regulatory consultants before. Innolitics is the first one that understood our software well enough to design a regulatory strategy from the architecture itself.
We needed a team that would get it done right the first time and independently. As a physician, I was delighted to work with another physician engineer on the team that was able to implement complex clinical workflows with very little input from me or my team. Innolitics delivered ahead of schedule and exceeded expectations. Every step of the way, Innolitics demonstrated why they’re leaders in medical software development.
We likely wouldn't have received our Breakthrough Device Designation without Innolitics. When FDA initially pushed back, they quickly understood the concerns and responded with clear, well-supported data. Their expertise in software, medicine, and FDA's AI/ML expectations made a decisive difference in the outcome.
Our FDA submission deadline was just two weeks away, and we had no software or cybersecurity documentation. We feared we would miss the deadline. Then our regulatory team introduced us to Innolitics. Their team swiftly validated our software and prepared the 15 necessary software and cybersecurity documents. They took a pragmatic approach that truly added value. This rapid timeline would have been impossible to meet without a team deeply knowledgeable in software, cybersecurity, AI/ML, and FDA regulations. In the end, we were able to submit on time! Thank you, Innolitics, for your Herculean efforts!
We recently got FDA cleared and Innolitics' responses were absolutely clutch to craft the strategy that finally worked. Unlike other consultants, who wanted us to do more work and spend more money on clinical testing, Innolitics found a path of least resistance using a combination of our existing validation and thoughtful responses to FDA. We received the best Christmas present ever – our 510(k) clearance letter.
We’re grateful to Innolitics for their expert help in getting our product 510(k) cleared. Our team was totally focused on other objectives, so we were excited to learn about the Innolitics Fast 510(k) service. Working with them was smooth, and now we have an FDA cleared product!
I needed a software development partner to write the software, train the AI, and get FDA clearance. An investor once told me that it would take me $5 million and 5 years to get to where we are now. Innolitics got me here 3 years ahead of schedule and $4 million dollars under budget.



Why digital health companies keep dying before and after FDA authorization, the evidence-reimbursement trap strangling the sector, and a post-marke...
Read more →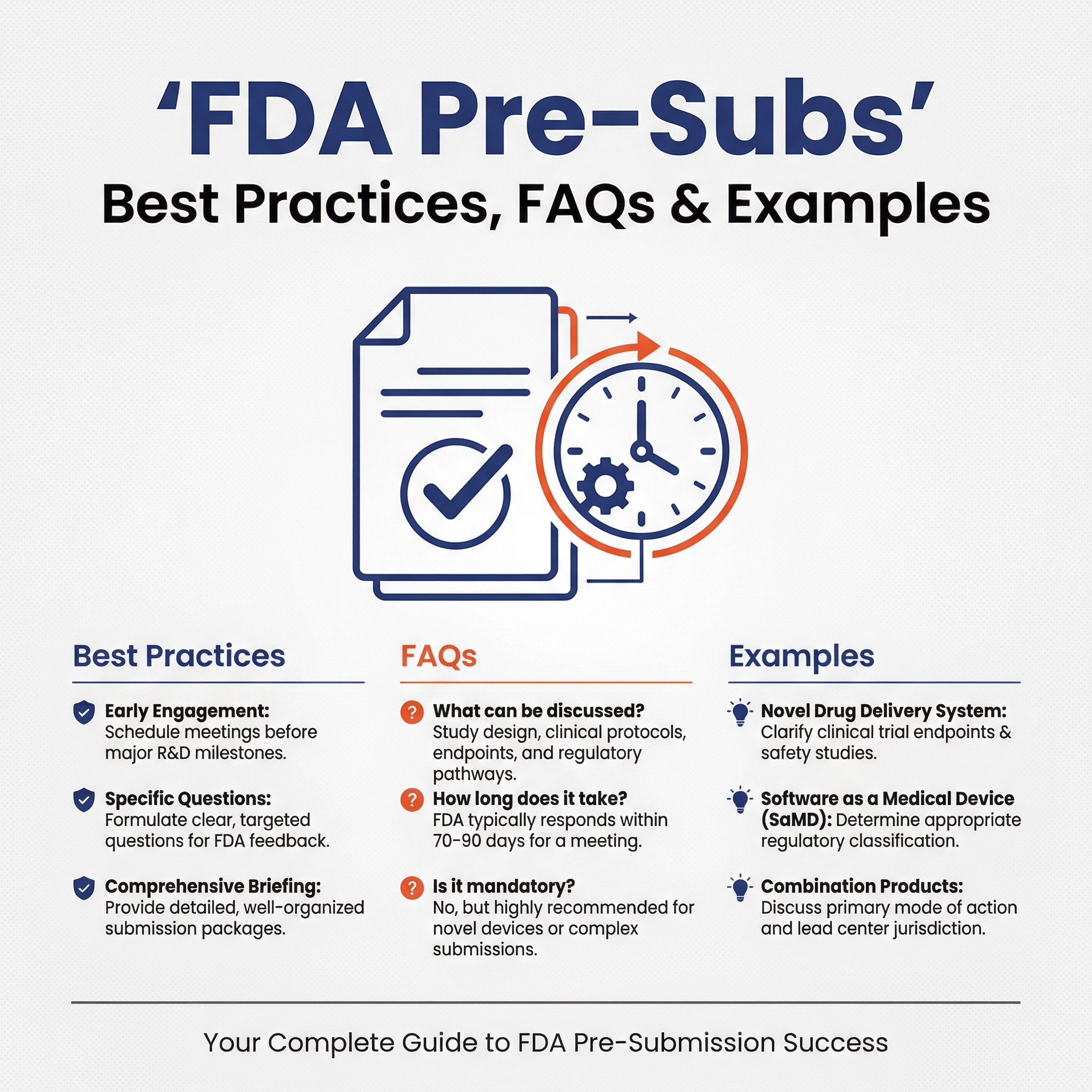
A Pre-Sub is a mechanism for requesting formal written feedback from the FDA, and (optionally) a one-hour meeting. Pre-subs are a useful means to m...
Read more →
AI-assisted medical device development is FDA-compliant when done right. The obstacle isn't regulation. It's the messy codebase you've been ignoring.
Read more →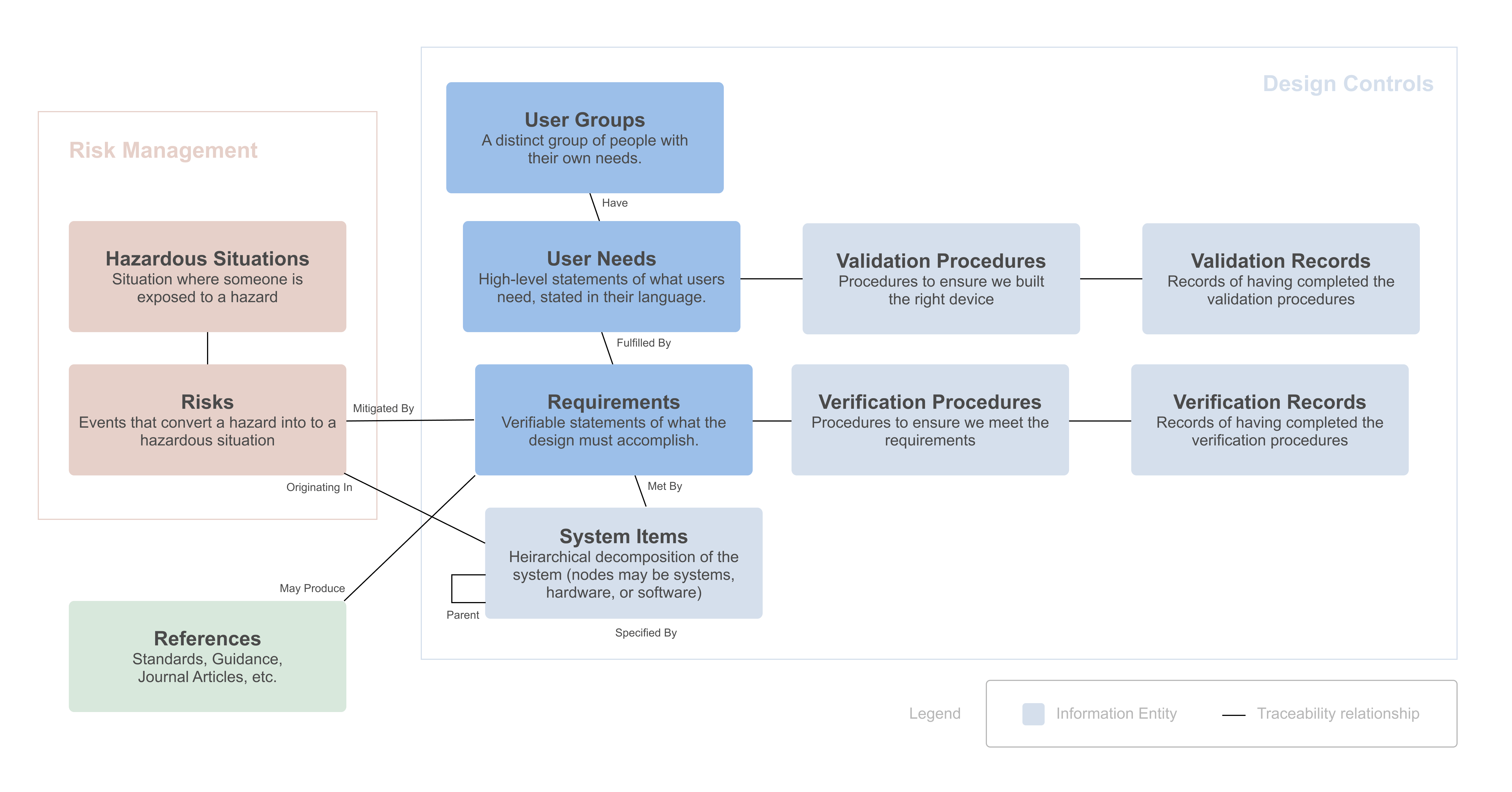
The FDA says developing your design inputs is "the single most important design control activity," yet writing good design inputs is difficult. Thi...
Read more →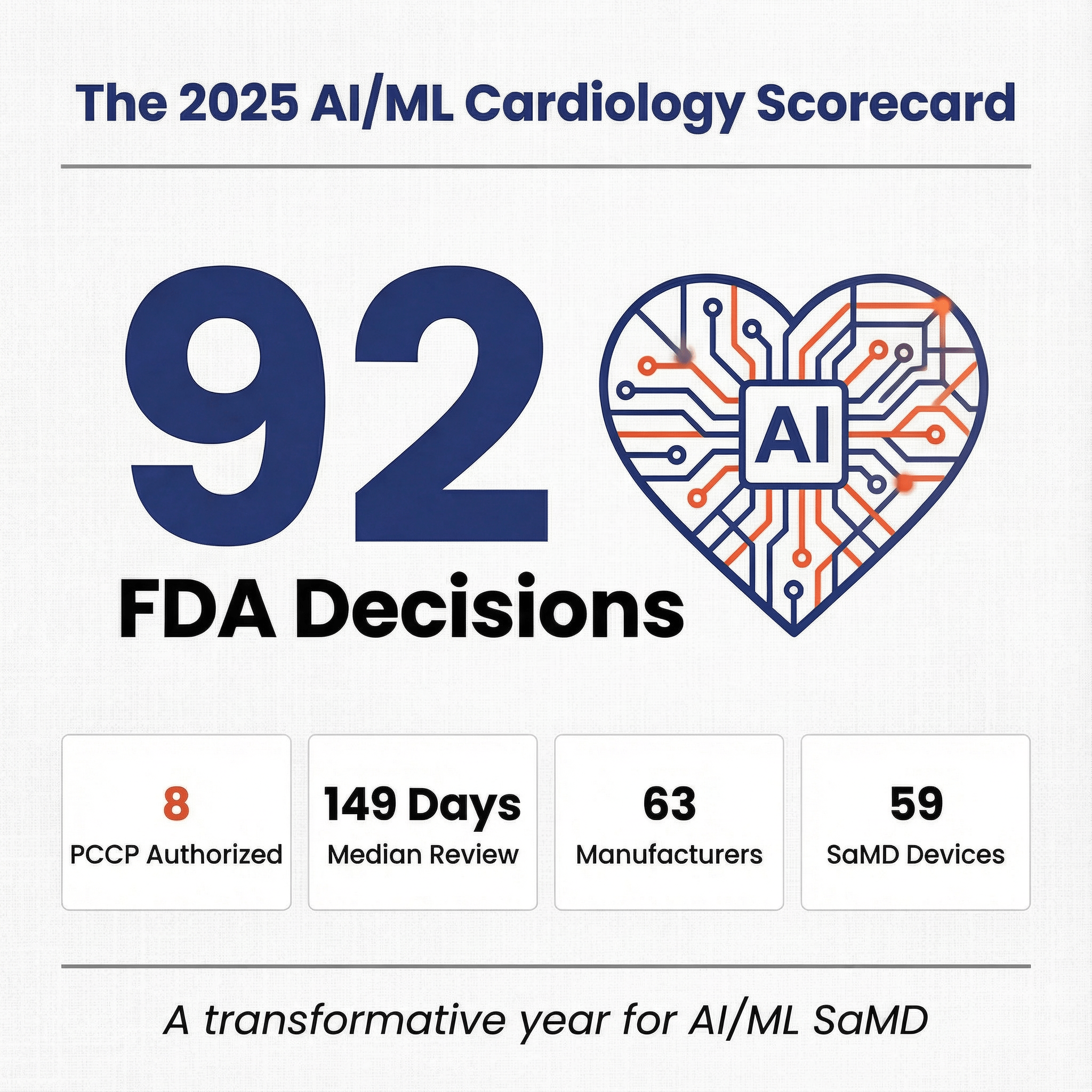
Comprehensive analysis of 2025 cardiology AI/ML clearances, market trends, capital events, and regulatory insights.
Read more →
A concise guide to the FDA's De Novo pathway for novel, moderate-risk medical devices that lack a suitable predicate. It includes real-world exampl...
Read more →
Side by side differences and executive takeaways from the January 2026 updates to the FDA CDS and General Wellness updates
Read more →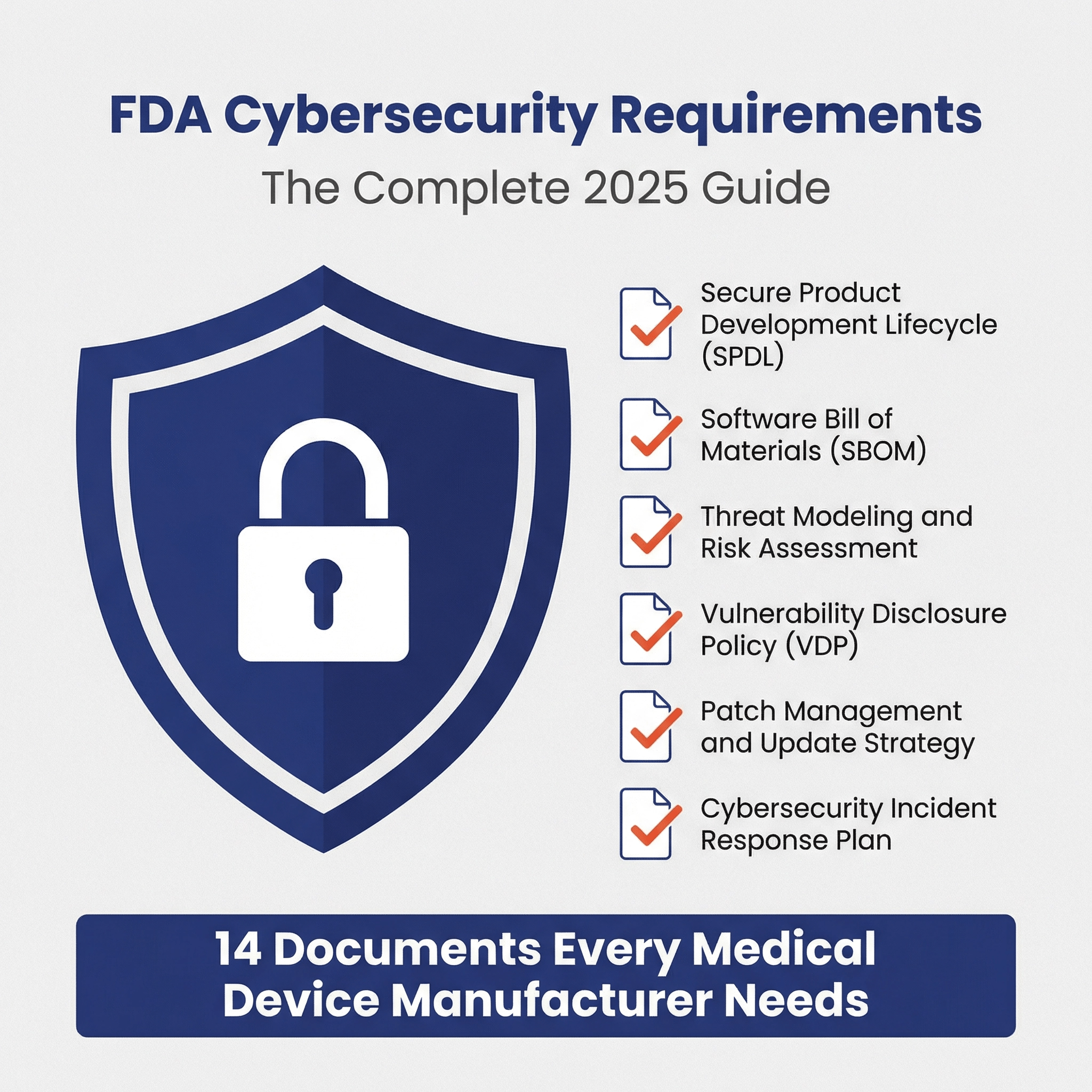
This article provides an in-depth exploration of medical device cybersecurity requirements, including best practices and FAQs. It also includes exa...
Read more →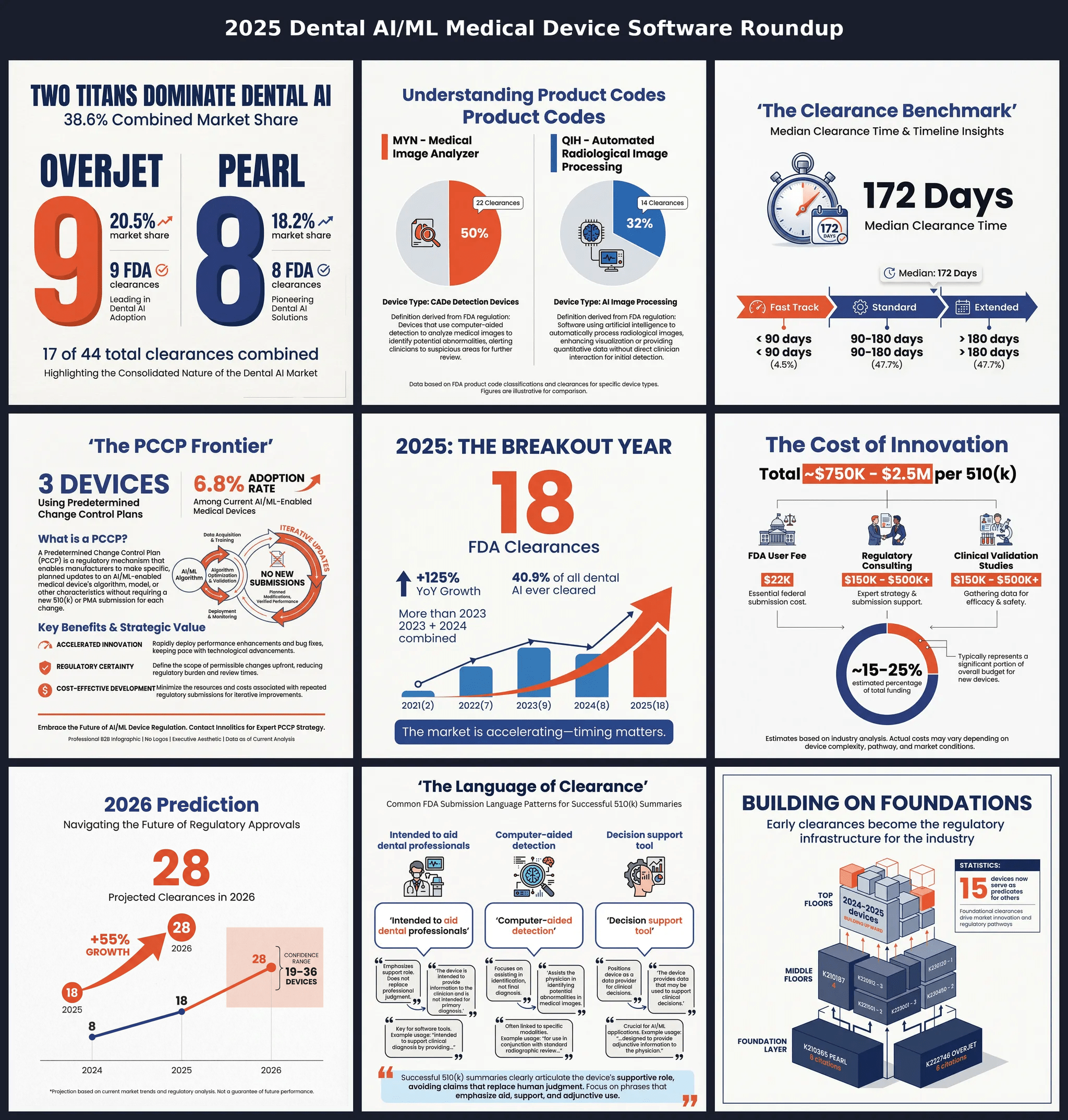
44 clearances. 18 in 2025 alone. The dental AI market has reached its tipping point. This report dissects the Pearl/Overjet duopoly controlling 34%...
Read more →
Practical suggestions and tips for authoring SBOMs for medical devices and for using them to monitor for cybersecurity vulnerabilities.
Read more →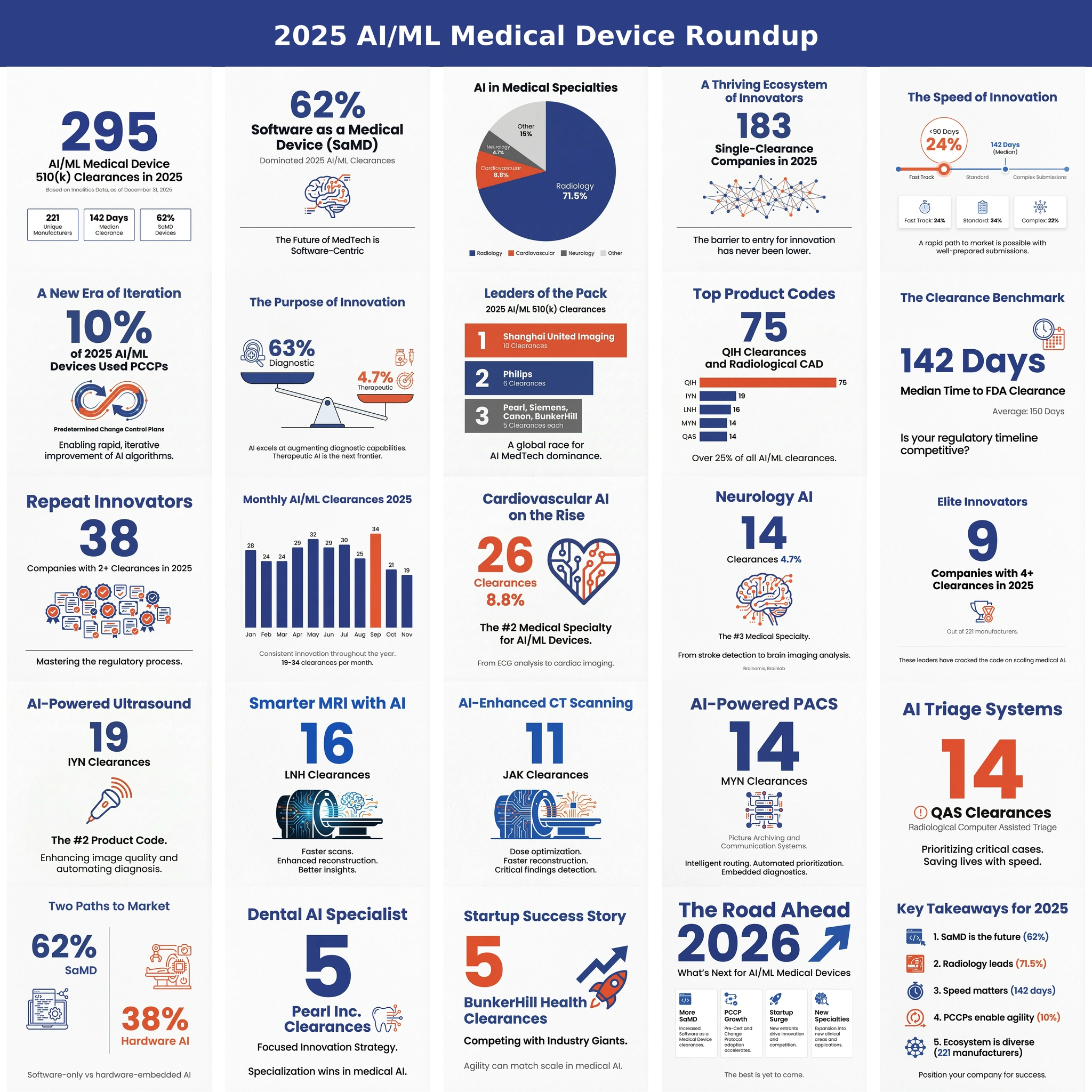
A data-driven look at 2025's AI/ML 510(k) clearances: volume, review times, SaMD share, leading specialties, top manufacturers, and early PCCP adop...
Read more →
A comprehensive FAQ for people who are interested in creating a new medical device software to learn the basics about the US regulations.
Read more →